文章信息
- 刘红娟, 吴仁杰, 谢水波, 刘迎九
- LIU Hong-juan, WU Ren-jie, XIE Shui-bo, LIU Ying-jiu
- 氧化石墨烯及其复合材料对水中放射性核素的吸附
- Graphene oxide and its composites for adsorption of radionuclides in water
- 材料工程, 2019, 47(10): 22-32
- Journal of Materials Engineering, 2019, 47(10): 22-32.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2018.001369
-
文章历史
- 收稿日期: 2018-11-23
- 修订日期: 2019-04-28
2. 南华大学 铀矿冶生物技术国防重点学科实验室, 湖南 衡阳 421001;
3. 南华大学 污染控制与资源化技术湖南省重点实验室, 湖南 衡阳 421001
2. Key Discipline Laboratory for National Defence for Biotechnology in Uranium Mining and Hydrometallurgy, University of South China, Hengyang 421001, Hunan, China;
3. Hunan Province Key Laboratory of Pollution Control and Resources Reuse Technology, University of South China, Hengyang 421001, Hunan, China
铀矿的开采和水冶、铀的精制和核燃料制造、反应堆运行及核燃料的后处理、放射性核素在医学上的应用等会产生放射性废水[1-2]。其进入生态环境之后,会通过食物链进入生物体。许多放射性核素如铯、铀、铕、和钍等进入生物体之后,能够产生电离辐射杀死细胞,妨碍正常细胞的分裂和再生,并导致细胞内遗传信息突变[3-4]。因此,对废水中的放射性核素进行有效去除已成为亟须解决的环境问题。目前,常用于放射性废水处理的技术包括化学沉淀法[5-7]、生物处理法[8-10]、离子交换法[11-12]、溶剂萃取法[13-14]和吸附法[15-19]等。其中吸附法由于具有低消耗、操作简单、效率高等特点,在处理放射性废水中具有潜在的应用价值[20-23]。对于吸附法而言,开发出比表面积大、稳定性好、效率高的新型吸附剂是非常重要的。近年来的研究表明,碳纳米管[24-27]、纳米碳[28-30]、氧化石墨烯(graphene oxide, GO)[31-37]等碳基纳米材料对放射性核素具有良好的吸附性能。GO是石墨烯的氧化物,具有结构独特、表面活性基团多、稳定性高等特点,在环境修复中受到广泛关注[34-35, 38]。Li等[38]研究发现, 在室温下pH=4时,GO对U(Ⅵ)的最大吸附容量为299mg·g-1。Wang等[39]研究发现,在pH=3.45和T=298K时,GO对U(Ⅵ)的最大吸附量达到0.396mmol·g-1。然而,由于GO片层之间强烈的相互作用,导致GO易趋向于自堆叠和不可逆聚集,这明显减小了GO的比表面积,从而妨碍了GO在废水处理中的吸附性能[40-42]。为了克服上述缺点,学者们将其他材料或功能基团接枝到GO表面,以改善GO的分散性和吸附性能。杨爱丽[43]研究发现,氧化石墨烯-壳聚糖复合吸附剂对铀的最大吸附量为114.94mg·g-1,而相同条件下GO对铀的最大吸附量仅为78.13mg·g-1。Wen等[44]研究了三维分层花状石墨烯氧化物羟基磷灰石复合材料对水中锶离子的去除,最大吸附容量能达到702.18mg·g-1,比GO高出9倍。本文对近年来氧化石墨烯及其复合材料对水中放射性核素吸附的研究现状及进展进行了综述,归纳了氧化石墨烯及其复合材料对放射性核素的吸附效果、影响因素和吸附机理等,并对其实际应用的前景进行了展望。
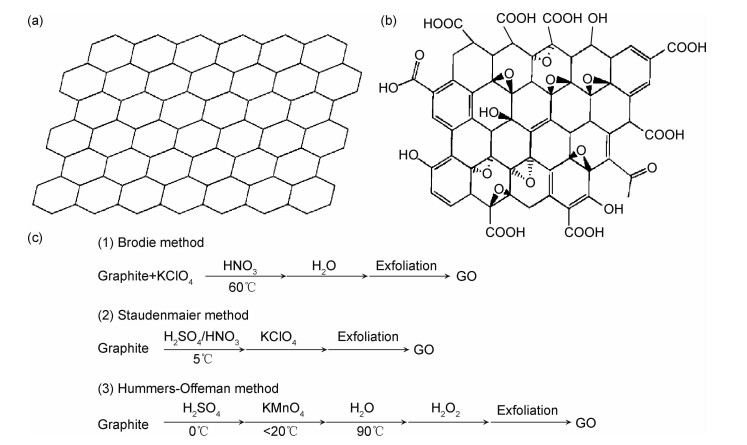
1 石墨烯和氧化石墨烯的结构和性质图 1为石墨烯和氧化石墨烯的结构图与氧化石墨烯的制备图。石墨烯(graphene,GN)(图 1(a))是一种由单原子层的碳原子通过sp2杂化,组成六角形呈蜂巢晶格状的二维材料[45],其理想厚度仅为0.335nm,理论比表面积高达2630m2·g-1,这些结构特性使其具有很多优异的性能,比如高热导率、良好的电学性能、高杨氏模量、断裂强度和快载流子迁移率等。这些独特的结构和优异的性能使其在能源、信息、材料、电子、环保和生物医学等方面具有潜在应用[46]。但是石墨烯由于相邻片层之间的相互作用,易发生团聚而重新堆叠成石墨,从而会阻碍其相关的研究与应用。目前研究者们主要利用GO和以GO为前驱体制备复合物。GO是单片层结构的石墨烯氧化物(图 1(b)),其二维基面上含有丰富的—COOH、—OH、羰基、环氧基等含氧基团。GO可以由化学方法制备得到,常用的化学氧化方法主要有Brodie法[47]、Staudenmaier法[48]以及Hummers法[49],其制备过程如图 1(c)所示[50]。Hummers法是目前较成熟的GO制备方法,该方法用浓硫酸和高锰酸钾混合物与天然石墨粉经氧化反应得到平面上有环氧基和酚羟基团以及边缘有衍生羧酸基的石墨薄片,再经超声剥离石墨薄片得到GO。GO可通过表面络合、配位、静电作用以及离子交换等来结合放射性核素,在污染物吸附方面具有优异的性能。
目前在石墨烯材料对放射性废水处理的研究方面,大多数集中于氧化石墨烯及其复合物。因为氧化石墨烯含有丰富的含氧基团,这些含氧基团能够有效地与放射性核素作用,因而其对放射性核素的吸附性能要优于石墨烯。目前学者们对氧化石墨烯及其复合材料吸附Cs,Sr,Eu,Th,Co和U等放射性核素进行了较多的研究,表 1汇总了氧化石墨烯及其复合材料在不同条件下对Cs,Sr,Eu,Th,Co和U六种放射性核素的吸附容量及主要参数[34-35, 42, 51-73]。研究发现,氧化石墨烯能有效吸附水中的这些放射性核素,且氧化石墨烯与其他物质复合制备得到的复合材料吸附性能都比氧化石墨烯的有所提高,但磁性氧化石墨烯的吸附性能有所降低。从表 1中的等温吸附和热力学研究发现,氧化石墨烯及其复合材料对水中放射性核素的吸附符合Langmiur模型,且为自发吸热的过程。
| Radionuclide | Adsorbent | pH value | T/K | Adsorption capacity/ (mg · g-1) | Isotherm model | Thermodynamics | Ref |
| Cs(Ⅰ) | GO | 6 | 298 | 180 | Langmiur | n.a. | [51] |
| Cs(Ⅰ) | GOs | 3 | 298 | 93.7 | n.a. | n.a. | [52] |
| Cs(Ⅰ) | PANI/GO | 3 | 298 | 190.43 | Langmiur | n.a. | [52] |
| Cs(Ⅰ) | Magnetic GO | 3 | 293 | 9.259 | Langmiur | Endothermic/spontaneous | [53] |
| Cs(Ⅰ) | PB/Fe3O4/GO | 4 | 298 | 55.5556 | Langmiur | Endothermic | [54] |
| Cs(Ⅰ) | PSMGPB | 7 | 303 | 437.03 | Langmiur | Endothermic/spontaneous | [55] |
| Cs(Ⅰ) | PFGMw(in water) | 7 | RT | 43.52 | Langmiur | Endothermic | [56] |
| Sr(Ⅱ) | GOs | 3 | 298 | 57.48 | Langmiur | n.a. | [52] |
| Sr(Ⅱ) | PANI/GO | 3 | 298 | 147.2 | Langmiur | n.a. | [52] |
| Sr(Ⅱ) | Magnetic GO | 4 | 293 | 14.706 | Langmiur | Endothermic/spontaneous | [53] |
| Sr(Ⅱ) | PAM/GO | 8.5 | 303 | 184.9 | Langmiur | Endothermic/spontaneous | [57] |
| Sr(Ⅱ) | Magnetic PANI/GO | 3 | 293 | 37.17 | Langmiur | n.a. | [58] |
| Sr(Ⅱ) | PAO-g-rGO | 8 | 303 | 99.4 | Langmiur | Endothermic/spontaneous | [59] |
| Sr(Ⅱ) | GO | 5 | 298 | 16.3 | Langmiur | n.a. | [35] |
| Sr(Ⅱ) | OGO | 8 | 303 | 85 | Langmiur | Endothermic/spontaneous | [60] |
| Eu(Ⅲ) | GOs | 3 | 298 | 144.82 | n.a. | n.a. | [52] |
| Eu(Ⅲ) | PANI/GO | 3 | 298 | 250.74 | Langmiur | n.a. | [52] |
| Eu(Ⅲ) | PAO-g-rGO | 5 | 303 | 296.4 | Langmiur | Endothermic/spontaneous | [59] |
| Eu(Ⅲ) | GONS | 6 | 298 | 175.44 | Langmiur | Endothermic/spontaneous | [34] |
| Eu(Ⅲ) | GOs | 4.5 | 298 | 89.65 | Langmiur | Endothermic/spontaneous | [61] |
| Eu(Ⅲ) | Magnetic GOs | 4.5 | 298 | 70.15 | Langmiur | Endothermic/spontaneous | [61] |
| Eu(Ⅲ) | GO-OSO3H | 5.5 | 293 | 125 | Langmiur | Endothermic/spontaneous | [62] |
| Eu(Ⅲ) | GO | 5.5 | 298 | 120.15 | Langmiur | Endothermic/spontaneous | [63] |
| Th(Ⅳ) | GO-PDA | 4 | 298 | 703 | Langmiur | Endothermic/spontaneous | [64] |
| Th(Ⅳ) | GO | 3.5 | 293 | 43.47 | Langmiur | Endothermic | [65] |
| Th(Ⅳ) | rGO | 3.5 | 293 | 62.1 | Langmiur | Endothermic | [65] |
| Th(Ⅳ) | GONF | 3.5 | 293 | 88.49 | Langmiur | Endothermic | [65] |
| Th(Ⅳ) | rGONF | 3.5 | 293 | 126.58 | Langmiur | Endothermic | [65] |
| Th(Ⅳ) | GO-S | 3 | 298 | 515.04 | Langmiur | n.a. | [66] |
| Co(Ⅱ) | GO | 6 | 303 | 106.3 | Langmiur | Endothermic/spontaneous | [67] |
| Co(Ⅱ) | PAO-g-rGO | 6 | 303 | 177.6 | Langmiur | Endothermic/spontaneous | [59] |
| U(Ⅵ) | CoFe2O4-rGO | 5 | 298 | 227.2 | Langmiur | Endothermic/spontaneous | [68] |
| U(Ⅵ) | GO-NH2 | 5 | 298.15 | 215.2 | Langmiur | Endothermic/spontaneous | [69] |
| U(Ⅵ) | HO-CB[6]/GO | 5.5 | 298 | 301.6 | Langmiur | Endothermic/spontaneous | [70] |
| U(Ⅵ) | GONRs | 6 | 298 | 394.1 | Langmiur | Endothermic/spontaneous | [71] |
| U(Ⅵ) | Fe3O4/GO | 6.8 | 298 | 28.32 | Langmiur | Endothermic/spontaneous | [72] |
| U(Ⅵ) | PAM/GO | 5 | 295 | 166.12 | Langmiur | Endothermic/spontaneous | [42] |
| U(Ⅵ) | MnO2-Fe3O4-rGO | 6 | 328 | 108.7 | Langmiur | Endothermic/spontaneous | [73] |
| U(Ⅵ) | GO-PDA | 5.5 | 298 | 718 | Langmiur | Endothermic/spontaneous | [64] |
| Note: n.a.is not application. | |||||||
当氧化石墨烯及其复合材料吸附放射性核素时,影响其吸附性能的因素主要有溶液pH值、反应温度、溶液离子强度和吸附时间等。
溶液pH值不仅影响氧化石墨烯及其复合材料表面官能团的质子化程度,还影响放射性核素在溶液中的化学性质和存在形态。放射性核素Eu,Sr,Cs,Co和U在水溶液中的存在形态随pH值变化而不同,从而导致吸附效果也不同。在pH<7时,Eu,Sr,Cs,Co在水溶液中一般以Eu3+和Eu(OH)2+,Sr2+,Cs+和Co2+形态存在; 在较低pH值时,由于H+与这些阳离子竞争吸附点位而减少吸附;随着pH值进一步升高,H+浓度降低从而减少竞争,同时吸附剂去质子化表面带负电,吸附剂与Eu3+和Eu(OH)2+,Sr2+,Cs+和Co2+离子静电引力增强而提高吸附量,通常在pH值为7~8时吸附量会达到最高值;随着pH继续升高,Eu3+,Sr2+,Cs+和Co2+会发生沉淀或共沉淀作用而继续保持高水平吸附量[35, 52-54, 56-63]。Kaewmee等[51]研究了pH值对GO吸附Cs+的影响,在pH值从1增加到12时,随着pH值的升高GO对Cs+的吸附效率增强。GO的表面含有丰富的官能团,当pH值增加时,这些官能团会更多去质子化而参与Cs+的吸附。Li等[53]研究了磁性氧化石墨烯对Sr(Ⅱ)和Cs(Ⅰ)的吸附,在pH值从2增加到6时,磁性氧化石墨烯对Sr(Ⅱ)和Cs(Ⅰ)的吸附量增加;在pH为7时,其对Sr(Ⅱ)和Cs(Ⅰ)的吸附量最高;当pH继续增大至11,吸附量能保持最高水平。在不同的pH值下,U(Ⅵ)在水溶液中的存在形态变化相对复杂,在pH<7时主要以UO22+,UO2OH+,(UO2)2(OH)22+和(UO2)3(OH)5+等阳离子形态存在,U(Ⅵ)的吸附量随pH值的变化趋势和Eu,Sr,Cs,Co类似。但当pH>7时,(UO2)3(OH)7—, UO2(OH)3—等阴离子形态产生,带负电的吸附剂会与铀酰阴离子产生静电斥力,从而降低铀的吸附量。文献[38, 60, 64, 70, 73-81]进行石墨烯基材料吸附铀的研究时发现相似的结论,在pH<7时,U(Ⅵ)的吸附量随着pH值升高而增加,在pH值为6~8时达到最大值,pH值继续增大吸附量反而下降。
反应温度也是影响吸附的重要因素之一。若随着反应温度的增加,放射性核素的吸附量增大,表明该吸附过程是吸热反应;若随着反应温度的增加,放射性核素的吸附量减少,则表明该吸附过程是放热反应。Li等[53, 61]和Li等[64]研究了不同石墨烯基复合材料对Sr(Ⅱ),Cs(Ⅰ),Eu(Ⅲ)和Th(Ⅳ)的吸附,结果表明,这些石墨烯基复合材料对Sr(Ⅱ),Cs(Ⅰ),Eu(Ⅲ)和Th(Ⅳ)的吸附随着温度升高而吸附能力增强,都为自发吸热的过程。Hu等[63]进行吸附热力学研究表明,GO对Eu(Ⅲ)的吸附也为自发吸热的过程,温度升高吸附增强。吴鹏等[82]研究了温度对四氧化三铁/氧化石墨烯纳米带复合材料吸附铀的影响,该材料对铀的吸附量随着温度的升高而提高,表明温度升高促进铀的吸附。由表 1可知,氧化石墨烯及其复合材料吸附放射性核素都为自发吸热的过程。温度能提高石墨烯基材料对放射性核素的吸附量,可能是由于反应温度的升高激活了吸附剂表面的活性位点,增加了氧化石墨烯及其复合材料的化学反应。另一方面,反应温度升高会改变放射性核素在溶液中的存在形态和扩散速率,从而提高吸附能力[83-84]。
溶液离子强度会影响氧化石墨烯及其复合材料双静电层的厚度, 从而引起该复合材料上吸附点位的变化,且可影响放射性核素在溶液中的存在形态,影响其对放射性核素的吸附能力[85]。Wang等[86]研究了GO对放射性废水中多种核素152+154Eu(Ⅲ),85+89Sr(Ⅱ),U(Ⅵ)和134Cs(Ⅰ)的吸附,GO对U(Ⅵ)和Eu(Ⅲ)吸附效果不受离子强度的影响,而对Sr(Ⅱ)和Cs(Ⅰ)的吸附效果有所影响,表明GO对U(Ⅵ)和Eu(Ⅲ)的吸附主要受内层络合作用,而对Sr(Ⅱ)和Cs(Ⅰ)的吸附受外层络合作用或离子交换作用。Li等[53]研究了离子强度对磁性氧化石墨烯吸附Sr(Ⅱ),Cs(Ⅰ)的影响。在pH<5时,磁性氧化石墨烯对Sr(Ⅱ),Cs(Ⅰ)的吸附效果随着离子强度的增加而增强;在pH>5时,磁性氧化石墨烯对Sr(Ⅱ)和Cs(Ⅰ)的吸附效果与离子强度无关。Sun等[34]和Hu等[63]研究发现,氧化石墨烯及其复合材料对Eu(Ⅲ)的吸附量几乎不受离子强度的影响,表明Eu(Ⅲ)在该复合材料上的吸附主要以内层表面络合为主。氧化石墨烯及其复合材料吸附放射性核素对溶液离子强度变化是否敏感,取决于复合材料和放射性核素的类型,对离子强度敏感[71, 87]和不敏感的情况均存在[34, 60, 63, 88-90]。
氧化石墨烯及其复合材料具有很大的比表面积,且表面具有丰富的官能团,这有利于提高其对放射性核素的吸附速率,通常在几十分钟至几小时就能达到吸附平衡。杨爱丽[43]研究发现,氧化石墨烯-壳聚糖复合吸附剂对铀的吸附在前20min即能达到较好的吸附效果,70min能达到吸附平衡,最大铀吸附效率为97.5%。Kaewmee等[51]研究了pH值为3和12时接触时间对GO吸附Cs+的影响,结果表明,铯能迅速吸附到GO上。吸附量在前10min迅速升高,在100min左右达到平衡吸附;在pH=12时,Cs+吸附达到平衡的速度比pH=3时要快。Hu等[63]观察到,在初始反应时间时Eu(Ⅲ)在GO上的吸附量显著增加,然后随着时间的增加而保持较高的水平。Eu(Ⅲ)=1.0mg·L-1时,大约97%的Eu(Ⅲ)在1h内被GO去除;Eu(Ⅲ)=10.0mg·L-1时,55%的Eu(Ⅲ)在10h内被GO去除。Zhao等[72]研究发现,5min之内HO-CB[6]/GO对铀的吸附效率可达到90%,且20min内达到吸附平衡。唐振平等[77]研究了氧化石墨烯/膨润土复合材料对U(Ⅵ)的吸附。在反应前5min吸附量急剧上升,5~120min后吸附量增加缓慢并最终达到平衡。通常,吸附速率随时间变化包括两个阶段:第一阶段为外表面吸附或瞬时吸附,该阶段吸附剂表面能提供很多可利用的吸附点位,吸附过程非常迅速;第二阶段为逐渐吸附,吸附过程比较缓慢,吸附速率主要受颗粒内扩散控制,直至达到吸附平衡[63, 75]。准一级动力学和准二级动力学模型常用于拟合放射性核素吸附量随时间变化的实验数据,以研究氧化石墨烯及其复合材料对放射性核素的吸附机理。文献报道[39, 53, 63-64, 69, 72, 89],氧化石墨烯及其复合材料吸附放射性核素的过程符合准二级动力学模型,表明该类材料对放射性核素的吸附主要为化学吸附过程。
4 氧化石墨烯及其复合材料对水中放射性核素的吸附机理不同的氧化石墨烯及其复合材料与放射性核素的相互作用机理不同,通常它们之间的作用机理包括静电引力、内层表面络合、外层表面络合、吸附沉淀、氧化还原反应等[80]。目前的研究一般采用吸附动力学、吸附等温模型、吸附热力学、表面络合模型、光谱分析技术和理论计算等对放射性核素在氧化石墨烯及其复合材料上的吸附机理进行探讨。
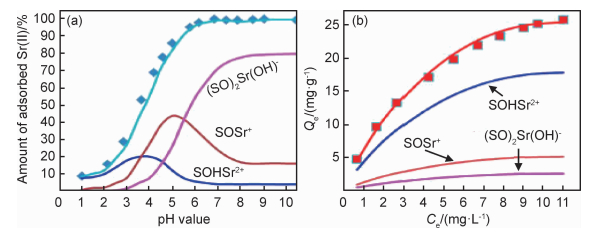
表面络合模型(surface complexation models,SCMs)通常包括三层模型(triple layer model, TLM)、恒定电容模型(constant capacitance model, CCM)和扩散层模型(diffuse layer model, DLM)。在研究石墨烯基材料与放射性核素固液界面的吸附机理时,SCMs作为一种有效的方法得到了很好的应用。Sun等[34]研究表明,SCMs能很好地拟合Eu(Ⅲ)在GOs上形成双核双齿配体(>SO)2Eu2(OH)22+配合物和单核单齿配体>SOEu2+配合物,该结论与离子强度依赖性吸附实验结果一致。在低pH值时,Eu(Ⅲ)在GOs上形成了>SOEu2+配合物,当pH值增大时(>SO)2Eu2(OH)22+配合物成了主要配合物。Hu等[58]利用DLM表面络合模型很好地模拟了PANI/GO复合材料对Sr(Ⅱ)的吸附中形成了外层络合物(SOHSr2+)和两种内球体配合((SO)2Sr(OH)-和SOSr+)(图 2)。Li等[61]采用DLM并在FITEQL 4.0软件的帮助下对Eu(Ⅲ)在GOs和磁性GOs上的吸附进行了研究,结果表明,在中性和高pH值条件下,Eu(Ⅲ)在磁性GOs上的吸附机理主要是单齿配位和多齿配位内层表面络合。于淑君[91]通过双扩散层表面络合模型拟合了U(Ⅵ)和Eu(Ⅲ)在GO上的吸附行为,得到单核单齿配合>SOM(n-1)+和>SOMOH(n-2)+络合物,Eu(Ⅲ)相对于U(Ⅵ)的lgK值较大。根据表面络合模型的拟合结果,可以得出放射性核素在GOs上的相对分布状态,帮助理解在不同pH值下放射性核素与GOs之间的相互作用机理。
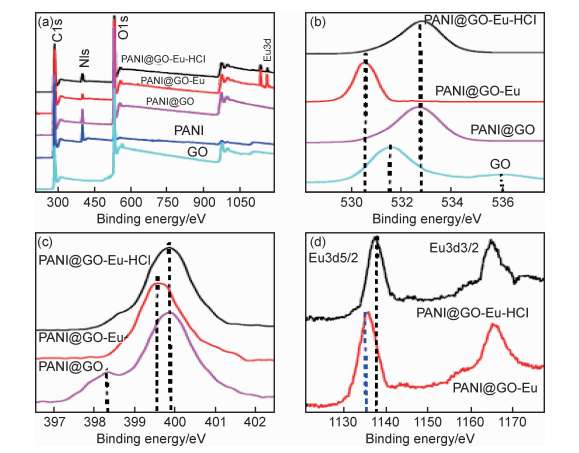
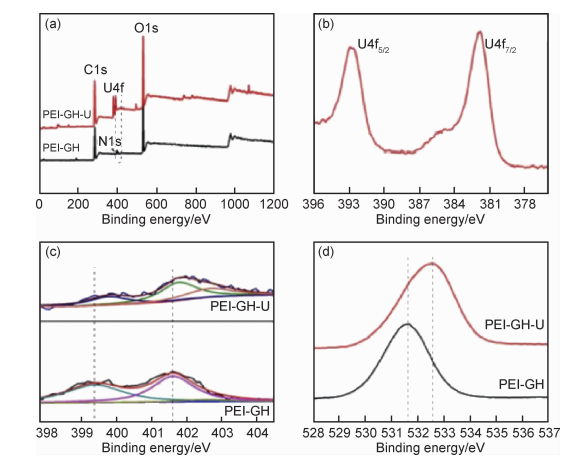
目前常用的光谱技术主要有傅里叶变换红外光谱法(Fourier transformed infrared spectroscopy, FTIR),X射线衍射(X-ray diffraction, XRD),扩展X射线吸收精细结构光谱(extended X-ray absorption fine structure spectroscopy, EXAFS),拉曼光谱(Raman spectroscopy, RS),X射线光电子能谱(X-ray photoelectric spectroscopy, XPS),荧光时间衰减光谱(time resolved laser fluorescence spectroscopy, TRLFS)等。这些光谱技术可分析放射性核素与材料之间形成的表面配合物的化学结构和特性,能够从分子水平揭示材料对放射性核素的吸附机理。Sun等[34]采用静态法和EXAFS法研究了GO纳米片对Eu(Ⅲ)的吸附机理,发现随pH值从6.3增至9.0,第一配位壳(Eu—O轨道)的配位数由6.69减少至6.02,Eu—C的键长也发生了改变,表明GO纳米片和Eu(Ⅲ)的作用机理主要为内层表面络合。Li等[38]通过EXAFS分析发现,GO上的含氧基团与UO22+之间形成了稳定的内表面络合物,从而为GO对UO22+有很强的作用力作出了解释。Sun等[52]通过化学氧化过程合成了PANI/GO复合材料,PANI/GO对Cs(Ⅰ),Sr(Ⅱ),U(Ⅵ)和Eu(Ⅲ)的吸附性能相比GO,活性炭和PANI有显著提高。XPS分析表明,PANI/GO纳米复合材料表面的含氧和含氮官能团是导致其高吸附能力的主要原因(图 3)。Hu等[58]结合静态法、EXAFS和表面络合模型探讨了Sr(Ⅱ)和磁性PANI/GO复合材料的作用机理,结果表明,当pH值为3时,磁性PANI/GO对Sr(Ⅱ)是外表面络合,而当pH值为7时对Sr(Ⅱ)是内表面络合。Wang等[81]利用FTIR和XPS对吸附铀前后的三维聚乙烯亚胺改性氧化石墨水凝胶进行了分析,结果表明,该水凝胶中的含氧官能团和氨基参与了对铀的配位(图 4)。从上述研究可以看到,光谱分析技术已发现GO基材料表面和边缘的功能基团能与放射性核素产生配位作用而实现高效吸附。然而,放射性核素在固液界面的行为表现复杂,受到其所在溶液环境、核素的性质与结构,以及材料的表面特性等多种因素的影响,还需要结合多种更先进的现代光谱分析技术,更深入系统地研究GO基材料对放射性核素的吸附机理。
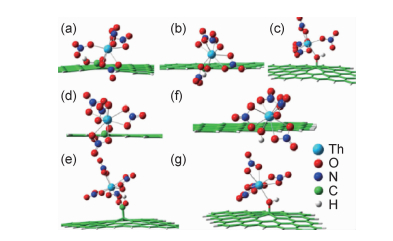
理论计算研究对于实验研究,尤其对那些由于条件的限制无法展开的实验研究,起到很重要的辅助作用。计算化学研究可以在分子水平上得到放射性核素于GO上所形成配合物的几何构型、电子结构、键合性质和分子性质,对固液界面吸附机理的研究有很重要的帮助。锕系元素配合物都是放射性和有毒的,尤其是超铀元素,对其进行实验研究相对困难,量子化学研究能为揭示锕系元素配合物的电子结构和键合性质等提供另一种有效途径[1]。Sun等[79]研究表明,不同功能化的GOs对U(Ⅵ)的吸附和解吸能力各不相同,密度泛函理论(density functional theory,DFT)计算结果表明,GOs上的羧基相比其他基团能够和U(Ⅵ)之间形成更强的配合物,DFT计算有助于从分子水平上理解GOs上不同官能团与铀的作用大小情况。文献[92-94]利用DFT研究了GO基材料与U(Ⅵ),Np(Ⅴ),Th(Ⅳ)和Pu(Ⅵ, Ⅳ)之间的配位模式与热力学行为,为阐明GO基材料与这些核素的相互作用机理提供重要的信息。Bai等[95]利用DFT研究了Th(Ⅳ)与羧基修饰的GO之间的相互作用。通过B3LYP方法优化三种Th(Ⅳ)/GO(COOH)和四种包含硝酸根离子的Th(Ⅳ)/GO(OH)结构(图 5)。三种Th(Ⅳ)/GO是八面体构型配合物,四种Th(Ⅳ)/GO是九面体构型配合物,所有的硝酸根离子都是双齿配体配位。对于这七种配合物,硝酸根阴离子的Th—O平均键距都在0.244~0.250nm范围内。羟基改性的Th—O键距要大于羧基改性的Th—O键距,表明羧基的配合能力比羟基强。Wu等[96]从理论上研究了GO基材料与Np(Ⅴ),Pu(Ⅵ)和Pu(Ⅳ)所形成配合物的结构、电子性质以及热力学行为,结果表明,相比Np(Ⅴ)和Pu (Ⅵ),Pu(Ⅳ)与GO基材料可以形成更多的配位键,GO基材料对这三种锕系元素的去除能力的顺序为Pu(Ⅳ)>Pu(Ⅵ)>Np(Ⅴ)。
上述的表面络合模型和光谱分析技术发现GO基材料对放射性核素的高效去除主要依靠其表面功能基团,然而GO基材料上存在多种功能基团,这些基团对配合放射性核素的贡献比例是表面络合模型和光谱分析技术难以揭示的。理论计算由于能提供放射性核素在水环境中配合物的形态分布、电子结构、配位模式和热力学等重要参数信息,计算出放射性核素与不同官能团的结合能,从而可以评估不同官能团对放射性核素配位的贡献大小,同时,理论计算也可以从分子水平判断GO基材料与不同放射性核素配位能力的强弱。
5 结束语氧化石墨烯由于其独特的二维结构、优异的物理和化学性质,其功能化改性及应用研究备受关注,学者们在其对放射性核素的去除效果、影响因素以及吸附去除机理等方面做了大量实验研究。然而,氧化石墨烯及其复合材料去除放射性核素还处于实验探索阶段,要推动这类材料在实际放射性废水处理中的工程应用,还面临诸多问题和挑战,主要有以下5方面:(1)辐射效应对氧化石墨烯及其复合材料结构稳定性和吸附去除性能的影响还需进行深入研究; (2)除了研究氧化石墨烯及其复合材料对单一放射性核素的吸附及机理,还需研究实际放射性废水中的共存组分体系的处理技术; (3)结合宏观批量实验、微观结构表征分析、数学模型和理论计算等手段多方位、深层次地探讨氧化石墨烯及其复合材料对放射性核素的吸附机理,以研制出高效、经济、选择性好的新型氧化石墨烯基材料; (4)氧化石墨烯基纳米材料大规模制备技术还亟须深入探索,要形成一套完善的该类材料的产业体系,实现生产的标准化、系列化和低成本化,以促进氧化石墨烯基材料在放射性废水处理中的实际应用; (5)积极研发与氧化石墨烯基材料相匹配的成套水处理工艺及设备,或者同目前成熟的放射性废水处理工艺相结合,扩大和提高氧化石墨烯基纳米材料在放射性废水处理中的应用范围和效率。
| [1] | WANG X X, YU S J, JIN J, et al. Application of graphene oxides and graphene oxide-based nanomaterials in radionuclide removal from aqueous solutions[J]. Science Bulletin, 2016, 61 (20): 1583–1593. DOI: 10.1007/s11434-016-1168-x |
| [2] |
曾勇. 环境核污染主要来源及其防治对策[J].
资源节约与环保, 2013 (4): 29–30.
ZENG Y. Major sources of environmental nuclear pollution and countermeasures[J]. Resources Economization & Environment Protection, 2013 (4): 29–30. DOI: 10.3969/j.issn.1673-2251.2013.04.018 |
| [3] | HU R, REN X, HOU G, et al. A carboxymethyl cellulose modified magnetic bentonite composite for efficient enrichment of radionuclides[J]. RSC Advances, 2016, 6 (69): 65136–65145. DOI: 10.1039/C6RA10990J |
| [4] |
杨姗也, 王祥学, 陈中山, 等. 四氧化三铁基纳米材料制备及对放射性元素和重金属离子的去除[J].
化学进展, 2018, 30 : 2205–242.
YANG S Y, WANG X X, CHEN Z S, et al. Synthesis of Fe3O4-based nanomaterials and their application in the removal of radionuclides and heavy metal ions[J]. Progress in Chemistry, 2018, 30 (Suppl 1): 2205–242. |
| [5] | TANG Y Z, REEDER R J. Uranyl and arsenate cosorption on aluminum oxide surface[J]. Geochimica et Cosmochimica Acta, 2009, 73 (10): 2727–2743. DOI: 10.1016/j.gca.2009.02.003 |
| [6] | SUN Y B, ZHANG R, DING C C, et al. Adsorption of U(Ⅵ) on sericite in the presence of Bacillus subtilis:a combined batch, EXAFS and modeling techniques[J]. Geochimica et Cosmo-chimica Acta, 2016, 180 : 51–65. DOI: 10.1016/j.gca.2016.02.012 |
| [7] | DING C, CHENG W, SUN Y, et al. Effects of Bacillus subtilis, on the reduction of U(Ⅵ) by nano-Fe0[J]. Geochimica et Cosmochimica Acta, 2015, 165 : 86–107. DOI: 10.1016/j.gca.2015.05.036 |
| [8] | GUPTA N K, SENGUPTA A, GUPTA A, et al. Biosorption-an alternative method for nuclear waste management:a critical review[J]. Journal of Environmental Chemical Engineering, 2018, 6 (2): 2159–2175. DOI: 10.1016/j.jece.2018.03.021 |
| [9] | LOVLEY D R, PHILLIPS E J P, GORBY Y A, et al. Microbial reduction of uranium[J]. Nature, 1991, 350 (6317): 413–416. DOI: 10.1038/350413a0 |
| [10] | WANG X, LIU Y, SUN Z, et al. Heap bioleaching of uranium from low-grade granite-type ore by mixed acidophilic microbes[J]. Journal of Radioanalytical & Nuclear Chemistry, 2017, 314 (1): 251–258. |
| [11] | BANERJEE C, DUDWADKAR N, TRIPATHI S C, et al. Nano-cerium vanadate:a novel inorganic ion exchange for removal of americium and uranium from simulated aqueous nuclear waste[J]. Journal of Hazardous Materials, 2014, 280 : 63–70. DOI: 10.1016/j.jhazmat.2014.07.026 |
| [12] | RENGARAJ S, MOON S H. Kinetics of adsorption of Co(Ⅱ) removal from water and wastewater by ion exchange resins[J]. Water Research, 2002, 36 (7): 1783–1793. DOI: 10.1016/S0043-1354(01)00380-3 |
| [13] | YUAN L Y, SUN M, LIAO X H, et al. Solvent extraction of U(Ⅵ) by trioctylphosphine oxide using a room-temperature ionic liquid[J]. Science China Chemistry, 2014, 57 (11): 1432–1438. DOI: 10.1007/s11426-014-5194-8 |
| [14] | RAO T P, METILDA P, GLADIS J M. Preconcentration techniques for uranium(Ⅵ) and thorium(Ⅳ) prior to analytical determination-an overview[J]. Talanta, 2006, 68 (4): 1047–1064. DOI: 10.1016/j.talanta.2005.07.021 |
| [15] | LIU X, CHENG C, XIAO C, et al. Polyaniline (PANI) modified bentonite by plasma technique for U(Ⅵ) removal from aqueous solution[J]. Applied Surface Science, 2017, 411 : 331–337. DOI: 10.1016/j.apsusc.2017.03.095 |
| [16] | JIN Q, SU L, MONTAVON G, et al. Surface complexation modeling of U(Ⅵ) adsorption on granite at ambient/elevated temperature:experimental and XPS study[J]. Chemical Geology, 2016, 433 : 81–91. DOI: 10.1016/j.chemgeo.2016.04.001 |
| [17] | LIU H, WANG R, JIANG H, et al. Study on adsorption characteristics of uranyl ions from aqueous solutions using zirconium hydroxide[J]. Journal of Radioanalytical & Nuclear Chemistry, 2015, 308 (1): 213–220. |
| [18] | ZHAO H, LIU X, YU M, et al. A study on the degree of amidoximation of polyacrylonitrile fibers and its effect on their capacity to adsorb uranyl ions[J]. Industrial & Engineering Chemistry Research, 2015, 54 (12): 3101–3106. |
| [19] | LIU H, XIE S, LIAO J, et al. Novel graphene oxide/bentonite composite for uranium(Ⅵ) adsorption from aqueous solution[J]. Journal of Radioanalytical & Nuclear Chemistry, 2018, 317 (3): 1349–1360. |
| [20] | PETRIE B, BARDEN R, KASPRZYK-HORDERN B. A review on emerging contaminants in wastewaters and the environment:current knowledge, understudied areas and recommendations for future monitoring[J]. Water Research, 2015, 72 : 3–27. DOI: 10.1016/j.watres.2014.08.053 |
| [21] | WANG S, MA M, MAN W, et al. One-step facile fabrication of sea urchin-like zirconium oxide for efficient phosphate sequestration[J]. RSC Adv, 2015, 5 : 91218–91224. DOI: 10.1039/C5RA12329A |
| [22] | WANG Y Q, ZHANG Z B, LIU Y H, et al. Adsorption of U(Ⅵ) from aqueous solution by the carboxyl-mesoporous carbon[J]. Chemical Engineering Journal, 2012, 198 (198/199): 246–253. |
| [23] | LI W P, HAN X Y, WANG X Y, et al. Recovery of uranyl from aqueous solutions using amidoximated polyacrylonitrile/exfoliated Na-montmorillonite composite[J]. Chemical Engi-neering Journal, 2015, 279 : 735–746. DOI: 10.1016/j.cej.2015.05.060 |
| [24] | WANG M, QIU J, TAO X, et al. Effect of pH and ionic strength on U(Ⅳ) sorption to oxidized multiwalled carbon nanotubes[J]. Journal of Radioanalytical & Nuclear Chemistry, 2011, 288 (3): 895–901. |
| [25] | FASFOUS I I, DAWOUD J N. Uranium (Ⅵ) sorption by multiwalled carbon nanotubes from aqueous solution[J]. Applied Surface Science, 2012, 259 (2): 433–440. |
| [26] | OUYANG J, WANG Y, LI T, et al. Immobilization of carboxyl-modified multiwalled carbon nanotubes in chitosan-based composite membranes for U(Ⅵ) sorption[J]. Journal of Radioanalytical & Nuclear Chemistry, 2018, 317 (3): 1419–1428. |
| [27] | WU J, TIAN K, WANG J. Adsorption of uranium (Ⅵ) by amidoxime modified multiwalled carbon nanotubes[J]. Progress in Nuclear Energy, 2018, 106 : 79–86. DOI: 10.1016/j.pnucene.2018.02.020 |
| [28] | DUBEY S, DWIVEDI A, SILLANPAA M, et al. Single-step green synthesis of imine-functionalized carbon spheres and their application in uranium removal from aqueous solution[J]. RSC Advances, 2014, 4 (86): 46114–46121. DOI: 10.1039/C4RA06890D |
| [29] | JIN H K, LEE H I, YEON J W, et al. Removal of uranium(Ⅵ) from aqueous solutions by nanoporous carbon and its chelating polymer composite[J]. Journal of Radioanalytical & Nuclear Chemistry, 2010, 286 (1): 129–133. |
| [30] | YUE Y F, SUN X G, MAYES R T, et al. Polymer-coated nanoporous carbons for trace seawater uranium adsorption[J]. Science China Chemistry, 2013, 56 (11): 1510–1515. DOI: 10.1007/s11426-013-4995-5 |
| [31] | HUANG G, PENG W, YANG S. Synthesis of magnetic chitosan/graphene oxide nanocomposites and its application for U(Ⅵ) adsorption from aqueous solution[J]. Journal of Radioanalytical & Nuclear Chemistry, 2018, 317 (2): 1–8. |
| [32] | YANG P, LIU Q, ZHANG H, et al. Phosphatidyl-assisted fabrication of graphene oxide nanosheets with multi-active sites for uranium(Ⅵ) capture[J]. Environmental Science-Nano, 2018, 5 (7): 1584–1594. DOI: 10.1039/C8EN00401C |
| [33] | BLIZNYUK V N, CONROY N A, XIE Y, et al. Increase in the reduction potential of uranyl upon interaction with graphene oxide surfaces[J]. Physical Chemistry Chemical Physics, 2018, 20 (3): 1752–1760. DOI: 10.1039/C7CP04197G |
| [34] | SUN Y, WANG Q, CHEN C, et al. Interaction between Eu(Ⅲ) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques[J]. Environmental Science & Technology, 2012, 46 (11): 6020–6027. |
| [35] | ROMANCHUK A Y, SLESAREV A S, KALMYKOV S N, et al. Graphene oxide for effective radionuclide removal[J]. Physical Chemistry Chemical Physics, 2013, 15 (7): 2321–2327. DOI: 10.1039/c2cp44593j |
| [36] | LI X, ZHAO K, YOU C, et al. Nanocomposites of polyaniline functionalized graphene oxide:synthesis and application as a novel platform for removal of Cd(Ⅱ), Eu(Ⅲ), Th(Ⅳ) and U(Ⅵ) in water[J]. Journal of Radioanalytical & Nuclear Chemistry, 2018, 315 (3): 509–522. |
| [37] | ZONG P, WANG S, ZHAO Y, et al. Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(Ⅵ) from aqueous solutions[J]. Chemical Engineering Journal, 2013, 220 (11): 45–52. |
| [38] | LI Z, CHEN F, YUAN L, et al. Uranium(Ⅵ) adsorption on graphene oxide nanosheets from aqueous solutions[J]. Chemical Engineering Journal, 2012, 210 (6): 539–546. |
| [39] | WANG C L, LI Y, LIU C L. Sorption of uranium from aqueous solutions with graphene oxide[J]. Journal of Radioanalytical & Nuclear Chemistry, 2015, 304 (3): 1017–1025. |
| [40] | HUANG Z, LI Z, WU Q, et al. Simultaneous elimination of cationic uranium(Ⅵ) and anionic rhenium(Ⅶ) by graphene oxide-poly(ethyleneimine) macrostructures:a batch, XPS, EXAFS, and DFT combined study[J]. Environmental Science Nano, 2018, 5 : 2077–2087. DOI: 10.1039/C8EN00677F |
| [41] | LI C, SHI G Q. Functional gels based on chemically modified graphenes[J]. Advanced Materials, 2014, 26 (24): 3992–4012. DOI: 10.1002/adma.201306104 |
| [42] | SONG W, WANG X, WANG Q, et al. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides[J]. Physical Chemistry Chemical Physics, 2015, 17 (1): 398–406. DOI: 10.1039/C4CP04289A |
| [43] |
杨爱丽. 氧化石墨烯-壳聚糖复合吸附剂的制备及其吸附性能[J].
稀有金属材料与工程, 2018, 47 (5): 1583–1587.
YANG A L. Preparation and adsorption properties of GO-chitosan composite adsorbent[J]. Rare Metal Materials and Engineering, 2018, 47 (5): 1583–1587. |
| [44] | WEN T, WU X, LIU M, et al. Efficient capture of strontium from aqueous solutions using graphene oxide-hydroxyapatite nanocomposites[J]. Dalton Transactions, 2014, 43 (20): 7464–7472. DOI: 10.1039/c3dt53591f |
| [45] | VAN NOORDEN R. Moving towards a graphene world[J]. Nature, 2006, 442 (7100): 228–229. DOI: 10.1038/442228a |
| [46] | XIANG Q, YU J, JARONIEC M. Graphene-based semic-onductor photocatalysts[J]. Chemical Society Reviews, 2012, 41 (2): 782–796. DOI: 10.1039/C1CS15172J |
| [47] | BRODIE B C. On the atomic weight of graphite[J]. Philosophical Transactions of the Royal Society of London, 2009, 149 (1): 249–259. |
| [48] | STAUDENMAIER L. Verfahren zur darstellung der graph-itsäure[J]. Berichte Der Deutschen Chemischen Gesellschaft, 2006, 31 (2): 1481–1487. |
| [49] | WILLIAM S H J, RICHARD E O. Preparation of graphitic oxide[J]. Journal of the American Chemical Society, 1958, 80 (6): 1339. DOI: 10.1021/ja01539a017 |
| [50] | WANG S, SUN H, ANG H M, et al. Adsorptive remediation of environmental pollutants using novel graphene-based nano-materials[J]. Chemical Engineering Journal, 2013, 226 (24): 336–347. |
| [51] | KAEWMEE P, MANYAM J, OPAPRAKASIT P, et al. Effective removal of cesium by pristine graphene oxide:performance, characterizations and mechanisms[J]. RSC Advances, 2017, 7 (61): 38747–38756. DOI: 10.1039/C7RA04868H |
| [52] | SUN Y B, SHAO D D, CHEN C L, et al. Highly efficient enrichment of radionuclides on graphene oxide supported polyaniline[J]. Environmental Science & Technology, 2013, 47 : 9904–9910. |
| [53] | LI D, ZHANG B, XUAN F. The sequestration of Sr(Ⅱ) and Cs(Ⅰ) from aqueous solutions by magnetic graphene oxides[J]. Journal of Molecular Liquids, 2015, 209 : 508–514. DOI: 10.1016/j.molliq.2015.06.022 |
| [54] | YANG H, SUN L, ZHAI J, et al. In situ controllable synthesis of magnetic prussian blue/graphene oxide nanocomposites for removal of radioactive cesium in water[J]. Journal of Materials Chemistry A, 2013, 2 (2): 326–332. |
| [55] | KADAM A A, JANG J, LEE D S. Facile synthesis of pectin-stabilized magnetic graphene oxide Prussian blue nanocomposites for selective cesium removal from aqueous solution[J]. Bioresource Technology, 2016, 216 : 391–398. DOI: 10.1016/j.biortech.2016.05.103 |
| [56] | YANG H, LI H, ZHAI J, et al. Magnetic prussian blue/graphene oxide nanocomposites caged in calcium alginate microbeads for elimination of cesium ions from water and soil[J]. Chemical Engineering Journal, 2014, 246 (16): 10–19. |
| [57] | QI H, LIU H, GAO Y. Removal of Sr(Ⅱ) from aqueous solutions using polyacrylamide modified graphene oxide composites[J]. Journal of Molecular Liquids, 2015, 208 : 394–401. DOI: 10.1016/j.molliq.2015.04.061 |
| [58] | HU B W, QIU M Q, HU Q Y, et al. Decontamination of Sr(Ⅱ) on magnetic polyaniline/graphene oxide composites:evidence from experimental, spectroscopic, and modeling investigation[J]. ACS Sustainable Chemistry & Engineering, 2017, 5 : 6924–6931. |
| [59] | CHEN H, SHAO D, LI J, et al. The uptake of radionuclides from aqueous solution by poly(amidoxime) modified reduced graphene oxide[J]. Chemical Engineering Journal, 2014, 254 (7): 623–634. |
| [60] | LIU X, WANG X, LI J, et al. Ozonated graphene oxides as high efficient sorbents for Sr(Ⅱ) and U(Ⅵ) removal from aqueous solutions[J]. Science China Chemistry, 2016, 59 (7): 869–877. DOI: 10.1007/s11426-016-5594-z |
| [61] | LI D, ZHANG B, XUAN F. The sorption of Eu(Ⅲ) from aqueous solutions by magnetic graphene oxides:a combined experimental and modeling studies[J]. Journal of Molecular Liquids, 2015, 211 : 203–209. DOI: 10.1016/j.molliq.2015.07.012 |
| [62] | YAO T, XIAO Y, WU X, et al. Adsorption of Eu(Ⅲ) on sulfonated graphene oxide:combined macroscopic and modeling techniques[J]. Journal of Molecular Liquids, 2016, 215 : 443–448. DOI: 10.1016/j.molliq.2015.11.030 |
| [63] | HU B, HU Q, LI X, et al. Rapid and highly efficient removal of Eu(Ⅲ) from aqueous solutions using graphene oxide[J]. Journal of Molecular Liquids, 2017, 229 : 6–14. DOI: 10.1016/j.molliq.2016.12.030 |
| [64] | LI F H, YANG Z, WENG H Q, et al. High efficient separation of U(Ⅵ) and Th(Ⅳ) from rare earth elements in strong acidic solution by selective sorption on phenanthroline diamide functionalized graphene oxide[J]. Chemical Engineering Journal, 2018, 332 : 340–350. DOI: 10.1016/j.cej.2017.09.038 |
| [65] | LINGAMDINNE L P, CHOI Y L, KIM I S, et al. Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides[J]. Journal of Hazardous Materials, 2016, 326 : 145–156. |
| [66] | PAN N, LI L, DING J, et al. A Schiff base/quaternary ammonium salt bifunctional graphene oxide as an efficient adsorbent for removal of Th(Ⅳ)/U(Ⅵ)[J]. Journal of Colloid and Interface Science, 2017, 508 : 303–312. DOI: 10.1016/j.jcis.2017.08.068 |
| [67] | ZHAO G, LI J, REN X, et al. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management[J]. Environmental Science & Technology, 2011, 45 (24): 10454–10462. |
| [68] | TAN L C, LIU Q, SONG D L, et al. Uranium extraction using a magnetic CoFe2O4-graphene nanocomposite:kinetics and thermodynamics studies[J]. New Journal of Chemistry, 2015, 39 (4): 2832–2838. DOI: 10.1039/C4NJ01981D |
| [69] | LIU S J, LI S, ZHANG H X, et al. Removal of uranium(Ⅵ) from aqueous solution using graphene oxide and its amine-functionalized composite[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 309 (2): 607–614. |
| [70] | SHAO L, ZHONG J R, REN Y M, et al. Perhydroxy-CB[J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 311 (1): 627–635. DOI: 10.1007/s10967-016-5067-z |
| [71] | WANG Y, WANG Z S, GU Z X, et al. Uranium(Ⅵ) sorption on graphene oxide nanoribbons derived from unzipping of multiwalled carbon nanotubes[J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 304 (3): 1329–1337. DOI: 10.1007/s10967-015-3981-0 |
| [72] | ZHAO D L, CHEN L L, SUN M, et al. Preparation and application of magnetic graphene oxide composite for the highly efficient immobilization of U(Ⅵ) from aqueous solutions[J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 306 (1): 221–229. DOI: 10.1007/s10967-015-4064-y |
| [73] | TAN L C, WANG J, LIU Q, et al. The synthesis of a manganese dioxide-iron oxide-graphene magnetic nanocomposite for enhanced uranium(Ⅵ) removal[J]. New Journal of Chemistry, 2015, 39 (2): 868–876. DOI: 10.1039/C4NJ01256A |
| [74] | ZHAO Z W, LI J X, WEN T, et al. Surface functionalization graphene oxide by polydopamine for high affinity of radionuclides[J]. Colloids & Surfaces a Physicochemical & Engineering Aspects, 2015, 482 : 258–266. |
| [75] | HAN R, ZOU W, WANG Y, et al. Removal of uranium(Ⅵ) from aqueous solutions by manganese oxide coated zeolite:discussion of adsorption isotherms and pH effect[J]. Journal of Environmental Radioactivity, 2007, 93 (3): 127–143. DOI: 10.1016/j.jenvrad.2006.12.003 |
| [76] | LIU S J, OUYANG J X, LUO J Q, et al. Removal of uranium(Ⅵ) from aqueous solution using graphene oxide functionalized with diethylenetriaminepentaacetic phenylenediamine[J]. Journal of Nuclear Science and Technology, 2018, 55 (7): 78–79. |
| [77] |
唐振平, 童克展, 谢水波, 等. 氧化石墨烯/膨润土复合材料对废水中铀(Ⅵ)的吸附试验研究[J].
科学技术与工程, 2018 (16): 175–180.
TANG Z P, TONG K Z, XIE S B, et al. Experimental study on adsorption of uranium(Ⅵ) in wastewater by graphene oxide/bentonite composites[J]. Science Technology and Engineering, 2018 (16): 175–180. DOI: 10.3969/j.issn.1671-1815.2018.16.027 |
| [78] | ZHAO D L, GAO X, CHEN S H, et al. Interaction between U(Ⅵ) with sulfhydryl groups functionalized graphene oxides investigated by batch and spectroscopic techniques[J]. Journal of Colloid and Interface Science, 2018, 524 : 129–138. DOI: 10.1016/j.jcis.2018.04.012 |
| [79] | SUN Y, YANG S, CHEN Y, et al. Adsorption and desorption of U(Ⅵ) on functionalized graphene oxides:a combined experimental and theoretical study[J]. Environmental Science & Technology, 2015, 49 (7): 4255–4262. |
| [80] | WANG Y N, LIU X, HUANG Y S, et al. Interaction mechanisms of U(Ⅵ) and graphene oxide from the perspective of particle size distribution[J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 311 (1): 209–217. DOI: 10.1007/s10967-016-4924-0 |
| [81] | WANG X, LIU Q, LIU J, et al. 3D self-assembly polyethyleneimine modified graphene oxide hydrogel for the extraction of uranium from aqueous solution[J]. Applied Surface Science, 2017, 426 : 1063–1074. DOI: 10.1016/j.apsusc.2017.07.203 |
| [82] |
吴鹏, 王云, 胡学文, 等. 四氧化三铁/氧化石墨烯纳米带复合材料对铀的吸附性能[J].
原子能科学技术, 2018, 52 (9): 1561–1568.
WU P, WANG Y, HU X W, et al. Uranium adsorption on ferroferric oxide/graphene oxide nanoribbon composite material[J]. Atomic Energy Science and Technology, 2018, 52 (9): 1561–1568. |
| [83] | GAO Y, CHEN K, TAN X L, et al. Interaction mechanism of Re(Ⅶ) with zirconium dioxide nanoparticles archored onto reduced graphene oxides[J]. ACS Sustainable Chemistry & Engineering, 2017, 5 (3): 2163–2171. |
| [84] | ZHAO D L, ZHANG Q, XUAN H, et al. EDTA functionalized Fe3O4/graphene oxide for efficient removal of U(Ⅵ) from aqueous solutions[J]. Journal of Colloid & Interface Science, 2017, 506 : 300–307. |
| [85] | HU R, SHAO D D, WANG X K. Graphene oxide/polypyrrole composites for highly selective enrichment of U(Ⅵ) from aqueous solutions[J]. Polymer Chemistry, 2014, 5 (21): 6207–6215. DOI: 10.1039/C4PY00743C |
| [86] | WANG X, CHEN Z, WANG X. Graphene oxides for simultaneous highly efficient removal of trace level radionuclides from aqueous solutions[J]. Science China Chemistry, 2015, 58 (11): 1766–1773. DOI: 10.1007/s11426-015-5435-5 |
| [87] | SONG W C, SHAO D D, LU S S, et al. Simultaneous removal of uranium and humic acid by cyclodextrin modified graphene oxide nanosheets[J]. Science China Chemistry, 2014, 57 (9): 1291–1299. DOI: 10.1007/s11426-014-5119-6 |
| [88] | ZHAO Y G, LI J X, ZHANG S W, et al. Efficient enrichment of uranium(Ⅵ) on amidoximated magnetite/graphene oxide composites[J]. RSC Advances, 2013, 3 : 18952–18959. DOI: 10.1039/c3ra42236d |
| [89] | ZHANG Z B, QIU Y F, DAI Y, et al. Synthesis and application of sulfonated graphene oxide for the adsorption of uranium(Ⅵ) from aqueous solutions[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 310 (2): 547–557. DOI: 10.1007/s10967-016-4813-6 |
| [90] | DING C C, CHENG W C, SUN Y B, et al. Determination of chemical affinity of graphene oxide nanosheets with radionuclides investigated by macroscopic, spectroscopic and modeling techniques[J]. Dalton Transactions, 2014, 43 : 3888–3896. DOI: 10.1039/C3DT52881B |
| [91] |
于淑君.石墨烯基材料与环境污染物相互作用机理研究[D].合肥: 中国科学技术大学, 2017. YU S J. Interaction mechanism between graphene-based mat-erials and environmental pollutants[D]. Hefei: University of Science and Technology of China, 2017. http://cdmd.cnki.com.cn/Article/CDMD-10358-1017174814.htm |
| [92] | XIAO C L, WU Q Y, WANG C Z, et al. Quantum chemistry study of uranium(Ⅵ), neptunium(Ⅴ), and plutonium(Ⅳ, Ⅵ) complexes with preorganized tetradentate phenanthrolineamide ligands[J]. Inorganic Chemistry, 2014, 53 (20): 10846–10853. DOI: 10.1021/ic500816z |
| [93] | LAN J H, SHI W Q, YUAN L Y, et al. Recent advances in computational modeling and simulations on the An(Ⅲ)/Ln(Ⅲ) separation process[J]. Coordination Chemistry Reviews, 2012, 256 (13/14): 1406–1417. |
| [94] | WU Q Y, LAN J H, WANG C Z, et al. Understanding the bonding nature of uranyl ion and functionalized craphene:a theoretical study[J]. Journal of Physical Chemistry A, 2014, 118 (11): 2149–2158. DOI: 10.1021/jp500924a |
| [95] | BAI Z Q, LI Z J, WANG C Z, et al. Interactions between Th(Ⅳ) and graphene oxide:experimental and density functional theoretical investigations[J]. RSC Advances, 2014, 4 (7): 3340–3347. DOI: 10.1039/C3RA45938A |
| [96] | WU Q Y, LAN J H, WANG C Z, et al. Understanding the interactions of neptunium and plutonium ions with graphene oxide:scalar-relativistic DFT investigations[J]. Journal of Physical Chemistry A, 2014, 118 (44): 10273–10280. DOI: 10.1021/jp5069945 |
 2019, Vol. 47
2019, Vol. 47