文章信息
- 赵海涛, 马瑞廷, 刘瑞萍
- ZHAO Hai-tao, MA Rui-ting, LIU Rui-ping
- 热分解法制备Ni0.5Zn0.5Fe2O4纳米颗粒
- Synthesis of Ni0.5Zn0.5Fe2O4 Nanoparticles by Thermal Decomposition Method
- 材料工程, 2017, 45(9): 81-85
- Journal of Materials Engineering, 2017, 45(9): 81-85.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2015.001062
-
文章历史
- 收稿日期: 2015-08-28
- 修订日期: 2017-01-08
镍锌铁氧体具有较高的饱和磁化强度,较大的磁导率,较小的矫顽力和较低的涡流损耗,是一种典型的软磁材料,被广泛应用于微波器件、变压器的核心材料以及磁记录系统[1-4]。
制备铁氧体的方法有很多,常见的方法主要有固相烧结法[5]、化学共沉淀法[6]、水热法[7]、溶胶-凝胶法[8]、多元醇法[9]和热分解法[10]等。在这些方法中热分解法具有其独特的优势,如产物纯度高,粒径大小可控,分布窄等,因此成为近年来研究的热点。Song等[11]以油酸铁为前驱体通过热分解法制备了立方形和球形的纳米Fe3O4,研究发现立方形的纳米颗粒具有更好的饱和磁化强度,较好的磁共振成像对比效果和磁热效应。Zhang等[12]通过高温热分解法制备油酸包覆的粒径为19nm的颗粒,研究发现油酸通过化学作用吸附在纳米颗粒的表面,形成单层结构,可以有效地减小粒子之间的相互作用,有利于得到单分散的纳米颗粒。Li等[13]采用热分解法成功制备了单分散粒径约为24nm的铁氧体,并研究了产物的磁性能,研究发现产物具有较高的饱和磁化强度(78.68A·m2/kg),室温下表现为超顺磁性。然而目前采用热分解法制备镍锌铁氧体的报道较少,本工作采用高温热分解法制备了纳米Ni0.5Zn0.5Fe2O4铁氧体,并研究了不同反应条件对纳米颗粒的形貌和粒径的影响。
1 实验材料与方法 1.1 Ni0.5Zn0.5Fe2O4纳米粒子的制备按照摩尔比为2:0.5:0.5准确称取乙酰丙酮铁、乙酰丙酮镍、乙酰丙酮锌,溶解到40mL的十八烯中,加入0.2mmol的三辛基氧化膦(TOPO),在室温下用高纯氩气将反应混合物脱气10min后加热至80℃搅拌0.5h,继续升温至120℃,注入一定比例的油酸(OA)和油铵(OAm),保温0.5h,之后升温至260℃,回流反应1h。停止加热冷却至室温后离心洗涤,最后将洗涤后的黑色沉淀物置于60℃真空干燥箱中干燥24h,得到Ni0.5Zn0.5Fe2O4纳米粒子。
1.2 试样的表征物相分析用PW-3040型X射线衍射仪,扫描范围2θ为20°~70°。利用Philips EM 420型透射电镜观察粉体的形貌。采用VSM-2000型振动样品磁强计分析产物的磁性能。
2 结果与分析 2.1 物相分析图 1为TOPO加入量为0.2mmol,260℃回流1h条件下制备的Ni0.5Zn0.5Fe2O4纳米颗粒的XRD图谱。由图 1可见,特征峰的位置在2θ=30.05°,35.39°,36.95°,43.07°,53.36°,57.13°和62.60°处,对应的晶面指数与PDF卡片08-0234 (Ni, Zn)Fe2O4的标准图谱中(220),(311),(222),(400),(422),(511) 和(440) 一致,表明所制备的纳米粒子是立方晶系尖晶石结构。衍射峰尖锐且无明显的杂质峰,说明产物的纯度较高,结晶性较好。衍射峰出现明显的宽化,表明样品的尺寸达到了纳米尺度。通过Scherrer公式计算晶粒尺寸:
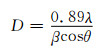
|
(1) |

|
图 1 Ni0.5Zn05.Fe2O4纳米粒子的XRD图谱 Fig. 1 XRD pattern of Ni0.5Zn0.5Fe2O4 nanoparticles |
式中:D为晶粒尺寸,nm;λ为X射线的波长,取0.154178nm;β为最强衍射峰的半高宽,弧度;θ为布拉格衍射角,(°)。选取图 1中的最强峰根据Scherrer公式计算得出纳米晶体的平均晶粒尺寸约为13.8nm。
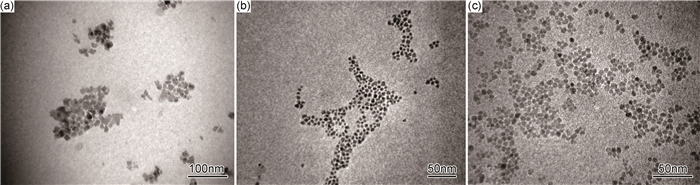
2.2 TOPO用量对粒子形貌的影响图 2为TOPO的加入量分别为0.2, 0.4mmol和0.6mmol条件下制备的Ni0.5Zn0.5Fe2O4纳米粒子的透射电镜照片。由图 2可见,不同TOPO用量下制备的颗粒粒径均较小,达到了纳米尺寸。随着TOPO用量的增加,纳米颗粒的粒径明显减小,从14nm减小到6nm,粒径分布变窄,分散性明显提高,颗粒形貌更加均一。这是因为TOPO作为表面活性剂吸附在纳米颗粒的表面,可以有效地阻碍颗粒的长大,使产物粒径减小,同时表面活性剂吸附在纳米颗粒表面还会产生空间位阻效应,有效地阻止颗粒因分子间相互作用力而发生团聚,提高产物的分散性。当TOPO用量较少时,表面活性剂不能完全包覆在颗粒表面,只能吸附在部分晶面上,使得不同晶面的生长速率不一致[14],产物的形貌不规则;随着TOPO用量增加,表面活性剂可以完全包覆在颗粒表面,形成高分散的近似球形的纳米颗粒。
|
图 2 不同TOPO用量制备的Ni0.5Zn0.5Fe2O4纳米粒子的透射电镜照片 (a)0.2mmol; (b)0.4mmol; (c)0.6mmol Fig. 2 TEM images of the synthesized Ni0.5Zn0.5Fe2O4 nanoparticles with various TOPO amounts (a)0.2mmol; (b)0.4mmol; (c)0.6mmol |
图 3为TOPO的加入量为0.6mmol,分别在240, 260℃和280℃下制备的Ni0.5Zn0.5Fe2O4纳米粒子的透射电镜照片。由图 3可见,不同回流温度下制备的纳米颗粒分散性较好,粒径分布窄,且随着回流温度升高颗粒粒径逐渐增加。这与纳米颗粒的成核生长机制有关[15]:回流温度较低时,成核过程占主导地位,大量成核使产物的粒径减小,随着回流温度升高,成核和生长过程发生竞争,成核和生长交替进行,使产物粒径增加,同时回流温度较高有利于晶粒生长,但是回流温度过高时,颗粒粒径也会明显增加,因此最佳反应温度应为260℃。

|
图 3 不同回流温度下制备的Ni0.5Zn0.5Fe2O4纳米粒子的透射电镜照片 (a)240℃; (b)260℃; (c)280℃ Fig. 3 TEM images of the synthesized Ni0.5Zn0.5Fe2O4 nanoparticles with various refluxing temperatures (a)240℃; (b)260℃; (c)280℃ |
图 4为TOPO的加入量为0.6mmol,在260℃下分别回流1, 1.5h和2h制备的Ni0.5Zn0.5Fe2O4纳米粒子的透射电镜照片。由图 4可以看出,不同回流时间下制备的Ni0.5Zn0.5Fe2O4纳米粒子分散性均较好,颗粒粒径较小、分布较窄。随着回流时间的延长,颗粒粒径增加,粒径分布变宽,形貌不均一。这是因为回流时间较短时,成核的纳米颗粒生长时间较短,产物粒径较小,随着回流时间延长,纳米颗粒之间发生二次成核或Ostwald熟化机制使产物粒径增加,颗粒尺寸不均一。

|
图 4 不同回流时间下制备的Ni0.5Zn0.5Fe2O4纳米粒子的透射电镜照片 (a)1h;(b)1.5h;(c)2h Fig. 4 TEM images of the synthesized Ni0.5Zn0.5Fe2O4 nanoparticles with various refluxing time (a)1h;(b)1.5h;(c)2h |
图 5为TOPO的加入量为0.6mmol,在260℃下回流1h所得的Ni0.5Zn0.5Fe2O4 纳米粒子室温(300K)下的磁滞回线。由图 5可见,产物的饱和磁化强度值(Ms)为49.38A·m2/kg,矫顽力(Hc)为7143.20A/m,剩余磁化强度(Mr)为5.76A·m2/kg,表现为亚铁磁性。产物的饱和磁化强度较大,矫顽力较小,这是因为制备的纳米镍锌铁氧体的结晶性高,形貌均一。然而纳米粒子的饱和磁化强度较传统的Ni0.5Zn0.5Fe2O4块体材料饱和磁化强度73A·m2/kg明显降低[16],这可能是因为纳米粒子表面自旋倾斜效应,以及反应过程中作为表面活性剂等非磁性物质TOPO吸附在粒子表面,形成非磁性层,降低了相同质量下磁性物质的含量,从而使产物的饱和磁性能降低[17]。

|
图 5 Ni0.5Zn0.5Fe2O4纳米粒子室温(300K)磁滞回线 Fig. 5 Magnetic hysteresis loop for the synthesized Ni0.5Zn0.5Fe2O4 nanoparticles at 300K |
镍锌铁氧体为典型的混合尖晶石结构铁氧体,其化学分子式可以表示为(ZnxFe1-x)[Ni1-xFe1+x]O4[18]其中()代表四面体间隙(A位),[]代表八面体间隙(B位)。铁氧体的磁性能与阳离子在间隙位置的分布密切相关[19],在正尖晶石结构ZnFe2O4铁氧体中,非磁性离子Zn2+全部占据四面体间隙(A位),磁性离子Fe3+全部占据八面体间隙(B位),这样导致A-B之间超交换作用较弱,饱和磁化强度较小。而在Ni0.5Zn0.5Fe2O4铁氧体中,Ni2+的加入会优先占据八面体间隙(B位),导致部分Fe3+从八面体间隙转移到四面体间隙中,使铁氧体中A-B间发生超交换作用的耦合离子数增多,A-B之间超交换作用增强,因此纳米Ni0.5Zn0.5Fe2O4铁氧体的饱和磁化强度明显增加[20]。
3 结论(1) 以乙酰丙酮金属盐为前驱体,十八烯为溶剂,采用热分解法制备了单分散的Ni0.5Zn0.5Fe2O4纳米颗粒,产物的粒径为6~14nm。
(2) 随着表面活性剂用量的增加,产物的粒径减小,分散性明显提高。回流温度提高,颗粒粒径增大。随着回流时间的延长,产物的粒径增加,粒径分布变宽,形貌不均一。最佳的生长条件:TOPO用量为0.6mmol,260℃回流1h。
(3) 最佳生长条件下制备的Ni0.5Zn0.5Fe2O4室温(300K)饱和磁化强度值为49.38A·m2/kg,矫顽力为7143.20A/m,剩余磁化强度为5.76A·m2/kg,表现为亚铁磁性。
| [1] | WANG L S, NIE S J, WANG J B, et al. Effect of experiment parameters on the structure and magnetic properties of NiZn-ferrite films[J]. Materials Chemistry and Physics, 2015, 160 : 321–328. DOI: 10.1016/j.matchemphys.2015.04.044 |
| [2] | WANG L, DONG H, LI J, et al. Effects of annealing temperature on structural and magnetic properties of Ni0.8Zn0.2Fe2O4 thin films[J]. Ceramics International, 2014, 40 (7): 10323–10327. DOI: 10.1016/j.ceramint.2014.03.004 |
| [3] | GUTIERREZ-LPEZA J, LEVENFELDA B, VAREZA A, et al. Study of the densification, mechanical and magnetic properties of Ni-Zn ferrites sintered in a solar furnace[J]. Ceramics International, 2015, 41 (5): 6534–6541. DOI: 10.1016/j.ceramint.2015.01.096 |
| [4] |
赵海涛, 张强, 刘瑞萍, 等. 单分散纳米锌铁氧体的制备及其磁性能[J].
材料工程, 2016, 44 (1): 103–107.
ZHAO H T, ZHANG Q, LIU R P, et al. Synthesis and magnetic properties of monodisperse ZnFe2O4 nanoparticles[J]. Journal of Materials Engineering, 2016, 44 (1): 103–107. DOI: 10.11868/j.issn.1001-4381.2016.01.016 |
| [5] | IBRAHIM I R, HASHIM M, NAZLAN R, et al. Grouping trends of magnetic permeability components in their parallel evolution with microstructure in Ni0.3Zn0.7Fe2O4[J]. Journal of Magnetism and Magnetic Materials, 2014, 355 : 265–275. DOI: 10.1016/j.jmmm.2013.12.024 |
| [6] | RASHAD M M, RAYAN D A, TURKY A O, et al. Effect of Co2+ and Y3+ ions insertion on the microstructure development and magnetic properties of Ni0.5Zn0.5Fe2O4 powders synthesized using Co-precipitation method[J]. Journal of Magnetism and Magnetic Materials, 2015, 374 : 359–366. DOI: 10.1016/j.jmmm.2014.08.031 |
| [7] |
曹慧群, 魏波, 刘剑洪, 等. 水热法制备纳米镍锌铁氧体粉体及其磁性能[J].
硅酸盐学报, 2007, 35 (6): 713–716.
CAO H Q, WEI B, LIU J H, et al. Hydrothermal synthesis and magnetic properties of nanosized nickel zinc ferrite powder[J]. Journal of the Chinese Ceramic Society, 2007, 35 (6): 713–716. |
| [8] |
张永刚, 徐波, 王树林, 等. 镍锌铁氧体纳米材料制备及其光催化性能研究[J].
功能材料, 2013, 44 (14): 2010–2013.
ZHANG Y G, XU B, WANG S L, et al. Preparation and photocatalytic properties of Ni-Zn ferrite nanopower[J]. Journal of Functional Materials, 2013, 44 (14): 2010–2013. DOI: 10.3969/j.issn.1001-9731.2013.14.008 |
| [9] |
张宁, 吴华强, 冒丽, 等. 微波多元醇法制备单分散Ni1-xZnxFe2O4/MWCNTs与磁性能[J].
功能材料, 2012, 43 (18): 2554–2557.
ZHANG N, WU H Q, MAO L, et al. Microwave-assisted polyol synthesis and magnetic properties of monodisperse Ni1-xZnxFe2O4/MWCNTs nanocomposites[J]. Journal of Functional Materials, 2012, 43 (18): 2554–2557. DOI: 10.3969/j.issn.1001-9731.2012.18.029 |
| [10] | ZHU Y, JIANG F Y, CHEN K X, et al. Size-controlled synthesis of monodisperse superparamagnetic iron oxide nanoparticles[J]. Journal of Alloys and Compounds, 2011, 509 (34): 8549–8553. DOI: 10.1016/j.jallcom.2011.05.115 |
| [11] | SONG M J, ZHANG Y, HU S L, et al. Influence of morphology and surface exchange reaction on magnetic properties of monodisperse magnetite nanoparticles[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2012, 408 : 114–121. |
| [12] | ZHANG L, HE R, GU H C. Oleic acid coating on the monodisperse magnetite nanoparticles[J]. Applied Surface Science, 2006, 253 (5): 2611–2617. DOI: 10.1016/j.apsusc.2006.05.023 |
| [13] | LI D, JIANG D L, CHEN M, et al. An easy fabrication of monodisperse oleic acid-coated Fe3O4 nanoparticles[J]. Materials Letters, 2010, 64 (22): 2462–2464. DOI: 10.1016/j.matlet.2010.08.025 |
| [14] |
李浩, 张喜斌. 络合剂/表面活性剂对溶剂热法制备钴纳米花的影响[J].
无机盐工业, 2010, 42 (6): 30–32.
LI H, ZHANG X B. Effect of complexing agents/surfactants on flowery Co microcrystals prepared by solvothermal method[J]. Inorganic Chemicals Industry, 2010, 42 (6): 30–32. |
| [15] |
陈瑾. 高温多元醇法制备超顺磁CoFe2O4纳米颗粒磁共振造影剂[J].
无机材料学报, 2009, 24 (5): 967–972.
CHEN J. High temperature polyol synthesis of superparamagnetic CoFe2O4 nanoparticles for magnetic resonance imaging contrast agents[J]. Journal of Inorganic Materials, 2009, 24 (5): 967–972. |
| [16] | ABBAS M, PARVATHEESWARA RAO B, KIM C G. Shape and size-controlled synthesis of Ni-Zn ferrite nanoparticles by two different routes[J]. Materials Chemistry and Physics, 2014, 147 (3): 443–451. DOI: 10.1016/j.matchemphys.2014.05.013 |
| [17] | HAJALILOUA A, HASHIM M, EBRAHIMI-KAHRIZSANGI R, et al. Synthesis and structural characterization of nano-sized nickel ferrite obtained by mechanochemical process[J]. Ceramics International, 2014, 40 (4): 5881–5887. DOI: 10.1016/j.ceramint.2013.11.032 |
| [18] | GHASEMIN A, MOUSAVINIA M. Structural and magnetic evaluation of substituted NiZnFe2O4 particles synthesized by conventional sol-gel method[J]. Ceramics International, 2014, 40 (2): 2825–2834. DOI: 10.1016/j.ceramint.2013.10.031 |
| [19] | LAZAREVIĆ Z Ž, MILUTINOVIĆ A N, JOVALEKIĆČ D, et al. Spectroscopy investigation of nanostructured nickel-zinc ferrite obtained by mechanochemical synthesis[J]. Materials Research Bulletin, 2015, 63 : 239–247. DOI: 10.1016/j.materresbull.2014.12.005 |
| [20] | WU H Q, ZHANG N, MAO L, et al. Controlled synthesis and magnetic properties of monodisperse Ni1-xZnxFe2O4/MWCNT nanocomposites via microwave-assisted polyol process[J]. Journal of Alloys and Compounds, 2013, 554 : 132–137. DOI: 10.1016/j.jallcom.2012.11.185 |
 2017, Vol. 45
2017, Vol. 45


