文章信息
- 谈玲华, 徐建华, 寇波, 杭祖圣, 石丽丽, 王钧
- TAN Ling-hua, XU Jian-hua, KOU Bo, HANG Zu-sheng, SHI Li-li, WANG Jun
- g-C3N4/NiO复合材料的制备及其对AP热分解的影响
- Preparation of g-C3N4/NiO Composites and Its Effect on Thermal Decomposition of Ammonium Perchlorate
- 材料工程, 2016, 44(11): 96-100
- Journal of Materials Engineering, 2016, 44(11): 96-100.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2016.11.016
-
文章历史
- 收稿日期: 2015-01-19
- 修订日期: 2016-03-13
2. 南京工程学院 材料工程学院, 南京 211167
2. School of Materials Science and Engineering, Nanjing Institute of Technology, Nanjing 211167, China
g-C3N4是类石墨结构的氮化碳材料,以三嗪环(C3N3环)或3-s-三嗪环(C6N7环)为结构单元,C, N原子均发生sp2杂化,通过pz轨道上的孤对电子形成一个类似于苯环结构的大π键,组成高度离域的共轭体系,层与层之间存在大量自由移动的电子[1, 2]。由于g-C3N4具有活性中心点多、化学稳定性好、耐高温、导电性能高、环境友好等特点[3, 4],作为新型的无金属催化剂备受关注,在环境、能源和化工等领域都有较好的应用前景[5, 6]。g-C3N4不仅可作为光催化剂用于光催化分解水制氢[7]、降解有机污染物[8]等方面,还可作为多相催化剂用于CO2活化反应[9]、Knoevenagel缩合反应[10]等方面[11]。与g-C3N4的光催化制氢及降解等方面的大量研究相比,在多相催化方面的研究报道相对较少。
据报道,为了提高g-C3N4的催化活性,可采用微观结构及形貌控制[12, 13]、N原子缺陷[14]、化学掺杂改性[15, 16]、物理复合改性[17-20]等方法。其中,g-C3N4与TiO2[17], WO3[18], SmVO4[19], ZnWO4[20]等金属氧化物材料复合能显著提高其光催化活性。
高氯酸铵(AP)是端羟基丁二烯(HTPB)复合固体推进剂中的高能组分,在推进剂中占60%~80%的比例,其热分解特性与推进剂的燃烧性能密切相关,通过研究催化剂对AP热分解的影响可推测推进剂的燃烧性能[21]。经前期研究,g-C3N4对AP的热分解表现出良好的催化作用,为了进一步提高g-C3N4的催化效果,将其与对AP有良好作用效果的NiO复合[22],探讨g-C3N4/NiO复合材料对AP热分解的催化效果,相关文献鲜见报道。
本工作拟采用混合煅烧法制备出g-C3N4/NiO复合材料,利用XRD, FT-IR, FESEM和EDS等对其进行表征,采用DTA和TG研究g-C3N4/NiO复合材料对AP热分解的影响,并探讨催化作用机理。
1 实验材料与方法三聚氰胺,国药化学试剂有限公司,分析纯;无水乙醇,国药化学试剂有限公司,分析纯;纳米NiO,南京艾普瑞纳米科技有限公司,40nm。
采用半封闭一步热解法[2]制备g-C3N4。取一定量的三聚氰胺放入陶瓷坩埚中(盖上坩埚盖),在马弗炉中以50℃/min升温到500℃,焙烧1h;5min内快速升温到520℃,保温焙烧1h,冷却研磨得g-C3N4粉末。
采用混合煅烧法[19]制备g-C3N4/NiO。取0.05g纳米NiO在乙醇中超声分散10min,然后加入0.95g g-C3N4继续超声分散10min,完成后在研钵中研磨至物体呈糊状,放入50℃真空烘箱中4h后,取出放入管式炉中,在300℃下焙烧1h得到g-C3N4/NiO复合材料。
将AP分别与g-C3N4,NiO,g-C3N4/NiO按照质量比为97:3的比例在一定量的乙醇溶液中混合、研磨,待乙醇挥发,干燥处理后得待测复合物(g-C3N4/NiO+AP)。
采用Ultima-IV型X射线衍射仪(XRD)分析样品的晶体结构,Kα辐射,波长为0.15406nm;采用NICOLET IS10型红外吸收光谱分析仪(FT-IR)进行红外分析,扫描范围400~4000cm-1;采用SU8010型场发射扫描电镜(FESEM)观察样品形貌,操作电压为30kV;采用GENESIS2000XMS60型X射线能谱仪(EDS)进行样品成分分析。
采用HTG-1型热分析仪(TGA)进行热失重分析,升温速率10℃/min,氮气流速20mL/min,试样量8mg左右,氧化铝样品池;采用404 PC型热分析仪(DTA)进行差热分析,升温速率10℃/min,氩气流速20mL/min,试样量10mg左右,氧化铝样品池。
2 结果与分析 2.1 物相分析对所制备的g-C3N4和g-C3N4/NiO复合材料进行XRD分析,结果如图 1所示。

|
图 1 g-C3N4,NiO和g-C3N4/NiO的XRD曲线 Fig. 1 XRD patterns of g-C3N4, NiO and g-C3N4/NiO |
由图 1可知,所制备的g-C3N4在2θ为13.2°和27.4°处出现两个较强的特征衍射峰,结合JCPDS 87-1526[23],分别对应于g-C3N4的(100)和(002)面。其中13.2°是melon类物质的特征峰,对应的晶面间距为0.675nm;而27.4°是典型的层状结构堆积衍射峰,对应的晶面间距为0.326nm[2]。g-C3N4/NiO复合材料既出现了g-C3N4的特征衍射峰,还在37.5°, 43.4°, 63.4°, 75.6°, 79.7°出现纳米NiO的衍射峰(JCPDS 47-1049) [24],说明所得的材料为g-C3N4/NiO复合材料。
图 2为g-C3N4和g-C3N4/NiO的FT-IR曲线。由图 2可知,纯g-C3N4在1200~1650cm-1之间出现吸收峰,1645cm-1处的吸收峰主要对应共轭CN的伸缩振动,1240, 1321, 1411, 1564cm-1处的吸收峰对应g-C3N4芳环结构的C-N旋转振动,807cm-1处的吸收峰则对应s-三嗪环的面外弯曲振动[25]。纳米NiO在646cm-1处出现红外吸收峰,对应Ni-O键的伸缩振动[26]。g-C3N4/NiO的FT-IR曲线中可以看出g-C3N4吸收峰较强,而NiO含量较低,其吸收峰相对较弱[27]。FT-IR也说明所制备的材料为g-C3N4/NiO复合材料。
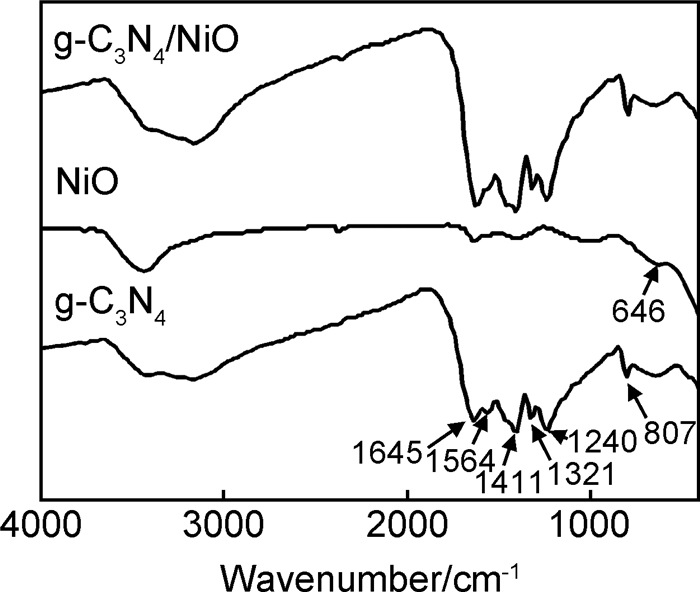
|
图 2 g-C3N4, NiO, g-C3N4/NiO的FT-IR曲线 Fig. 2 FT-IR patterns of g-C3N4, NiO and g-C3N4/NiO |
采用场发射扫描电子显微镜(FESEM)进一步观察g-C3N4/NiO微观形貌及结构,结果如图 3所示。
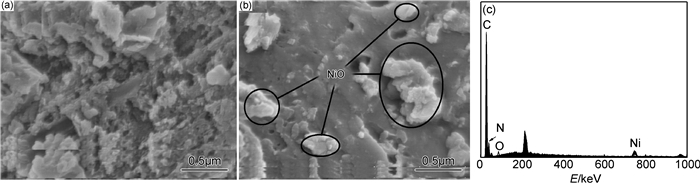
|
图 3 g-C3N4(a), g-C3N4/NiO (b)的FESEM图像及EDS曲线(c) Fig. 3 FESEM images of g-C3N4(a), NiO/g-C3N4(b) and EDS curve of the selected area (c) |
由图 3可知,通过半封闭一步热解法制备得到的g-C3N4样品具有明显的层状结构,比较疏松[16]。图 3(b)为g-C3N4/NiO的FESEM图片,其中亮点为纳米NiO,比较均匀地分散于g-C3N4的表面。对其进行EDS分析,结果如图 3(c)所示,出现C,N,Ni,O的特征峰,由于g-C3N4/NiO中NiO的含量很少,所以Ni元素的特征峰比较低。FESEM和EDS结果说明NiO与g-C3N4复合,并均匀分布于g-C3N4的表面。
2.2 对AP热分解的催化性能研究采用DTA和TG研究g-C3N4, NiO, g-C3N4/NiO对AP热分解的影响,结果如图 4所示。
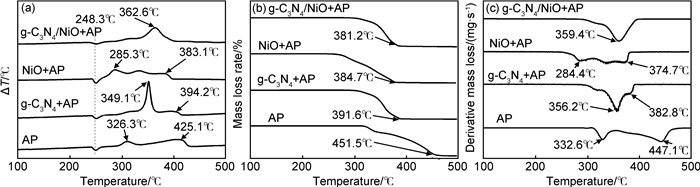
|
图 4 纯AP, g-C3N4+AP和g-C3N4/NiO+AP的DTA (a), TG (b), DTG (c)曲线 Fig. 4 Curves of DTA (a), TG (b) and DTG (c) of pure AP, AP mixed with g-C3N4 and g-C3N4/NiO |
由图 4可知,DTA曲线(如图 4(a)所示)有1个吸热峰和2个放热峰,248.3℃的吸热峰为AP由斜方晶系转变为立方晶系,326.3℃和425.1℃分别对应于AP的低温分解阶段高温分解和高温分解阶段[28]。添加g-C3N4, NiO, g-C3N4/NiO对AP的晶型转变没有影响,但却均能使AP的高温分解温度降低,对AP的热分解有促进作用。单独添加g-C3N4和NiO时,高温分解温度分别降低30.9℃和42.0℃,而加入g-C3N4/NiO后,AP的高温分解峰和低温分解峰合并,在362.6℃急剧分解,分解温度比纯AP降低了62.5℃,说明g-C3N4/NiO复合材料对AP的热分解起到较强的催化作用[29]。g-C3N4/NiO催化效果均比g-C3N4或NiO单独使用时强,说明g-C3N4和NiO具有协同催化作用[30]。纯AP的TG曲线(如图 4(b)所示)中出现两个失重平台,说明纯AP的热分解过程分两步进行。分别加入g-C3N4,NiO或g-C3N4/NiO后,AP的完全分解温度均有一定降低。根据图 4(c)的DTG曲线可知,纯AP在332.6℃和447.1℃出现失重速率极值。单独添加g-C3N4和NiO后,失重仍然是两个阶段,第二阶段失重对应温度降低,说明单一的g-C3N4和NiO对AP的热分解也具有催化作用。加入g-C3N4/NiO仅出现一个较大的失重峰,说明在这一阶段内快速分解,分解温度比纯AP的第二分解阶段降低了87.7℃,显示出较强的催化效果,其效果优于单独使用g-C3N4或NiO,也说明g-C3N4和NiO具有协同催化作用[30]。
在低温分解过程中,AP经质子转移离解生成气相的NH3和HClO4,HClO4(g)进一步分解生成氧化性中间产物C1O3, ClO, O, H2O等,氧化性中间产物如自由O与部分NH3发生氧化反应[22]。g-C3N4具有类石墨型层状结构,比表面积较大,有利于吸附NH3,HClO4等反应分子。但g-C3N4在AP表面覆盖程度大,阻碍了AP的离解与升华,抑制AP的低温分解,使低温分解温度增加。高温分解阶段是主要的分解阶段,该阶段不仅在气相中进行ClO4-氧化NH3的反应,也在凝聚相表面发生AP的分解过程。由于g-C3N4是离域的π共轭电子结构,具有非常优异的导电性能,有利于电子的转移和传导[31];NiO为P型半导体,d轨道可以提供良好的电子转移轨道,对AP热分解的电子转移过程起到桥接作用,有利于电子的转移[32];在g-C3N4/NiO复合材料中,g-C3N4与NiO形成异质结[33],具有更强的电子转移和传导能力,在氧化还原循环中进一步加速电子转移,使AP在更低的温度下分解。
3 结论(1) 采用混合煅烧法制备出g-C3N4/NiO复合材料,NiO均匀分散于g-C3N4的表面。
(2) g-C3N4/NiO复合材料使AP的高低温分解峰合并,高温分解温度降低了62.5℃,对AP的热分解表现出良好催化作用。g-C3N4/NiO的催化效果优于单独使用g-C3N4或NiO,g-C3N4和NiO具有协同催化作用。
| [1] | ALGARA-SILLER G, SEVERIN N, CHONG S Y, et al. Triazine-based graphitic carbon nitride: a two-dimensional semiconductor[J]. Angewandte Chemie,2014, 126 (29) : 7580 –7585. DOI: 10.1002/ange.201402191 |
| [2] | WANG X, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials,2008, 8 (1) : 76 –80. |
| [3] | XU J, BRENNER T J, CHABANNE L, et al. Liquid-based growth of polymeric carbon nitride layers and their use in a mesostructured polymer solar cell with Voc exceeding 1 V[J]. Journal of the American Chemical Society,2014, 136 (39) : 13486 –13489. DOI: 10.1021/ja508329c |
| [4] | LI X, WANG Y, KANG L, et al. A novel, non-metallic graphitic carbon nitride catalyst for acetylene hydrochlorination[J]. Journal of Catalysis,2014, 311 : 288 –294. DOI: 10.1016/j.jcat.2013.12.006 |
| [5] | ZHANG J, ZHANG M, YANG C, et al. Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface[J]. Advanced Materials,2014, 26 (24) : 4121 –4126. DOI: 10.1002/adma.v26.24 |
| [6] | ZHENG Y, LIN L, YE X, et al. Helical graphitic carbon nitrides with photocatalytic and optical activities[J]. Angewandte Chemie International Edition,2014, 53 (44) : 11926 –11930. DOI: 10.1002/anie.201407319 |
| [7] | SCHWINGHAMMER K, MESCH M B, DUPPEL V, et al. Crystalline carbon nitride nanosheets for improved visible-light hydrogen evolution[J]. Journal of the American Chemical Society,2014, 136 (5) : 1730 –1733. DOI: 10.1021/ja411321s |
| [8] | YAN S C, LI Z S, ZOU Z G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine[J]. Langmuir,2009, 25 (17) : 10397 –10401. DOI: 10.1021/la900923z |
| [9] | HUANG Z, LI F, CHEN B, et al. Well-dispersed g-C3N4 nanophases in mesoporous silica channels and their catalytic activity for carbon dioxide activation and conversion[J]. Applied Catalysis B: Environmental,2013, 136-137 : 269 –277. DOI: 10.1016/j.apcatb.2013.01.057 |
| [10] | TALAPANENI S N, ANANDAN S, MANE G P, et al. Facile synthesis and basic catalytic application of 3D mesoporous carbon nitride with a controllable bimodal distribution[J]. Journal of Materials Chemistry,2012, 22 (19) : 9831 –9840. DOI: 10.1039/c2jm30229b |
| [11] | WANG Y, WANG X, ANTONIETTI M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry[J]. Angewandte Chemie International Edition,2012, 51 (1) : 68 –89. DOI: 10.1002/anie.201101182 |
| [12] | SU F, MATHEW S C, LIPNER G, et al. mpg-C3N4-catalyzed selective oxidation of alcohols using O2 and visible light[J]. Journal of the American Chemical Society,2010, 132 (46) : 16299 –16301. DOI: 10.1021/ja102866p |
| [13] | MA T Y, DAI S, JARONIEC M, et al. Graphitic carbon nitride nanosheet-carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts[J]. Angewandte Chemie International Edition,2014, 53 (28) : 7281 –7285. DOI: 10.1002/anie.201403946 |
| [14] | NIU P, YIN L, YANG Y, et al. Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies[J]. Advanced Materials,2014, 26 (47) : 8046 –8052. DOI: 10.1002/adma.v26.47 |
| [15] | LIU G, NIU P, SUN C, et al. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4[J]. Journal of the American Chemical Society,2010, 132 (33) : 11642 –11648. DOI: 10.1021/ja103798k |
| [16] | LI X, CHEN J, WANG X, et al. Metal-free activation of dioxygen by graphene/g-C3N4 nanocomposites: functional dyads for selective oxidation of saturated hydrocarbons[J]. Journal of the American Chemical Society,2011, 133 (21) : 8074 –8077. DOI: 10.1021/ja200997a |
| [17] | HUANG Z A, SUN Q, LV K, et al. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs (101) facets of TiO2[J]. Applied Catalysis B: Environmental,2015, 164 : 420 –427. DOI: 10.1016/j.apcatb.2014.09.043 |
| [18] | CHEN S, HU Y, MENG S, et al. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3[J]. Applied Catalysis B: Environmental,2014, 150-151 : 564 –573. DOI: 10.1016/j.apcatb.2013.12.053 |
| [19] | LI T, ZHAO L, HE Y, et al. Synthesis of g-C3N4/SmVO4 composite photocatalyst with improved visible light photocatalytic activities in RhB degradation[J]. Applied Catalysis B: Environmental,2013, 129 : 255 –263. DOI: 10.1016/j.apcatb.2012.09.031 |
| [20] | SUN L, ZHAO X, JIA C, et al. Enhanced visible-light photocatalytic activity of g-C3N4-ZnWO4 by fabricating a heterojunction: investigation based on experimental and theoretical studies[J]. Journal of Materials Chemistry,2012, 22 (44) : 23428 –23438. DOI: 10.1039/c2jm34965e |
| [21] | LIU L L, LI F S, TAN L H, et al. Effects of nanometer Ni, Cu, Al and NiCu powders on the thermal decomposition of ammonium perchlorate[J]. Propellants, Explosives, Pyrotechnics,2004, 29 (1) : 34 –38. DOI: 10.1002/(ISSN)1521-4087 |
| [22] | 谈玲华, 李勤华, 杭祖圣, 等. 负载型纳米NiO催化高氯酸铵热分解的DSC/TG-MS研究[J]. 功能材料,2011, 42 (3) : 564 –567. TAN L H, LI Q H, HANG Z S, et al. Catalytic effect of supported nanometer NiO on the thermal decomposition of ammonium perchlorate by DSC/TG-MS[J]. Journal of Functional Materials,2011, 42 (3) : 564 –567. |
| [23] | SHI H, CHEN G, ZHANG C, et al. Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel[J]. ACS Catalysis,2014, 4 (10) : 3637 –3643. DOI: 10.1021/cs500848f |
| [24] | KIM H, JEONG H, KIM T, et al. Enhanced ethanol sensing characteristics of In2O3-decorated NiO hollow nanostructures via modulation of hole accumulation layers[J]. ACS Applied Materials & Interfaces,2014, 6 (20) : 18197 –18204. |
| [25] | ZHANG J, CHEN X, TAKANABE K, et al. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization[J]. Angewandte Chemie International Edition,2010, 49 (2) : 441 –444. DOI: 10.1002/anie.200903886 |
| [26] | VIJAYAKUMAR S, NAGAMUTHU S, MURALIDHARAN G. Supercapacitor studies on NiO nanoflakes synthesized through a microwave route[J]. ACS Applied Materials & Interfaces,2013, 5 (6) : 2188 –2196. |
| [27] | HUANG L, XU H, LI Y, et al. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalytic activity[J]. Dalton Transactions,2013, 42 (24) : 8606 –8616. DOI: 10.1039/c3dt00115f |
| [28] | BOLDYREV V V. Thermal decomposition of ammonium perchlorate[J]. Thermochimica Acta,2006, 443 (1) : 1 –36. DOI: 10.1016/j.tca.2005.11.038 |
| [29] | ZHANG W, LI P, XU H, et al. Thermal decomposition of ammonium perchlorate in the presence of Al (OH)3·Cr (OH)3 nanoparticles[J]. Journal of Hazardous Materials,2014, 268 : 273 –280. DOI: 10.1016/j.jhazmat.2014.01.016 |
| [30] | SUN J, YUAN Y, QIU L, et al. Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light[J]. Dalton Transactions,2012, 41 (22) : 6756 –6763. DOI: 10.1039/c2dt12474b |
| [31] | THOMAS A, FISCHER A, GOETTMANN F, et al. Graphitic carbon nitride materials: variation of structure and morphology and their use as metal-free catalysts[J]. Journal of Materials Chemistry,2008, 40 (9) : 4893 –4908. |
| [32] | 谈玲华, 李勤华, 杭祖圣, 等. 纳米NiO/MgO的制备及其对AP热分解催化性能影响[J]. 固体火箭技术,2011, 34 (2) : 214 –219. TAN L H, LI Q H, HANG Z S, et al. Preparation of nanometer NiO/MgO and its catalytic performance for thermal decomposition of ammonium perchlorate[J]. Journal of Solid Rocket Technology,2011, 34 (2) : 214 –219. |
| [33] | GAO Y, WANG L, LI Z, et al. Preparation of MXene-Cu2O nanocomposite and effect on thermal decomposition of ammonium perchlorate[J]. Solid State Sciences,2014, 35 : 62 –65. DOI: 10.1016/j.solidstatesciences.2014.06.014 |
 2016, Vol. 44
2016, Vol. 44


