文章信息
- 王国材, 肖小波, 陈艳萍, 王晨
- WANG Guo-cai, XIAO Xiao-bo, CHEN Yan-ping, WANG Chen
- Ce68Al10Cu20Nb2大块非晶表面钝化膜的研究
- Passive Film Formed on Ce68Al10Cu20Nb2 Bulk Amorphous Alloy
- 材料工程, 2016, 44(5): 72-78
- Journal of Materials Engineering, 2016, 44(5): 72-78.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2016.05.012
-
文章历史
- 收稿日期: 2014-09-28
- 修订日期: 2015-11-24
近年来,大块非晶合金由于具有高强度、高弹性极限和高断裂韧性等特性,已成为材料学界的研究热点[1, 2, 3]。在各种块体非晶合金体系中,Ce基大块非晶合金具有优异的玻璃形成能力和极低的玻璃转变温度(低于100℃),在沸水中可以像塑料一样进行复杂的变形加工,具有极高的科学研究价值和广阔的应用前景[4, 5]。自Ce基非晶被开发以来,人们对其结构[6, 7, 8],物理力学性能[9, 10, 11],玻璃形成能力[12, 13]等方面展开了广泛的研究。如Pelletier等[14]发现,(Ce0.72Cu0.28)90-xAl10Fex大块非晶合金的玻璃化转变温度(Tg)和晶化温度(Tx)随Fe含量的增加而升高。Yu等[15]发现Ce基大块非晶合金的屈服强度随着外界温度的降低显著增大。Zhou等[13]发现适当降低Ce原料(用于制备Ce基非晶合金)的纯度可以提高Ce-Ga-Cu大块非晶合金的玻璃形成能力。
Ce基大块非晶合金的耐腐蚀性能,直接关系到它的实际应用和应用前景。目前,已有关于Ti基、Ni基等大块非晶合金耐腐蚀性方面的报道[16, 17],但是有关Ce基非晶合金耐腐蚀性方面的研究报道极少,日本的Inoue[18]小组已经做了抗氧化性方面的工作,发现Zn的添加可以提高Ce-Cu-Al非晶合金的抗氧化性,但这也只是一个开端。Zhang等[4]发现Ce-Al-Cu-Nb合金具有优异的玻璃形成能力,其中Ce68Al10Cu20Nb2棒状试样的最大直径可以达到8mm以上[4]。但到目前为止,鲜见Ce-Al-Cu-Nb大块非晶合金有关腐蚀方面的报道。本工作采用电化学工作站、扫描电子显微镜和X射线光电子能谱仪等,发现Ce-Al-Cu-Nb大块非晶合金在NaOH溶液中具有表面钝化现象,研究了大块非晶表面钝化膜的形成过程、微结构和元素分布。
1 实验实验选用高纯度金属Ce(99.5%,质量分数,下同),Cu(99.995%),Al(99.999%)和Nb(99.999%)为原料,按照Ce68Al10Cu20Nb2(原子分数)的成分配比,利用真空电弧炉熔炼成合金铸锭,然后通过铜模冷铸法得到尺寸为1mm×8mm×30mm的板状非晶合金试样。测试前,将试样用 1200#,2000#,3000#SiC砂纸逐级打磨,然后抛光至表面光亮,分别用丙酮和酒精超声波清洗,冷风吹干后用于测试。
利用X射线衍射(XRD,D/max Ultima Ⅲ,CuKα)分析晶体结构。采用扫描电子显微镜(SEM,Zeiss Supra55)观察试样电化学钝化前后的微观形貌。采用X射线光电子能谱(XPS,ESCALAB-250)分析合金表面化学成分和元素化学状态,Ar+剥离束的能量为 3eV,电流密度为1μA/mm2,刻蚀速率为2.5nm/min。
电化学实验采用三电极体系,参比电极为氧化汞电极,本工作中电位都是相对于氧化汞电极,辅助电极为铂片,面积为2cm×2cm。实验采用CHI-660D的电化学工作站进行测试,实验前先测量开路电位,将试样放入1mol/L的NaOH 溶液中浸泡,待开路电位稳定后再进行极化曲线和交流阻抗谱(EIS)的测量。极化曲线的扫描速率为0.5mV/s,电势范围为-0.55~1.30V。交流阻抗谱测量时,交流正弦激励信号幅值为5mV,测试频率范围为1×10-2~1×105Hz,实验温度 25℃。根据极化曲线结果,将试样在-0.1V下恒电位钝化30min,得到用于分析的钝化膜。
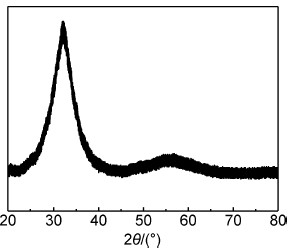
2 结果与讨论图 1为铜模快速凝固后制得的Ce68Al10Cu20Nb2合金的XRD图谱,可以看出,合金为单一的非晶态结构。Zhang等[4]在Ce基非晶合金的研究中也报道了类似的结果。
|
图1 铜模快速凝固后制得的Ce68Al10Cu20Nb2合金的XRD图谱 Fig.1 XRD pattern of Ce68Al10Cu20Nb2 bulk alloy solidified in copper mold |
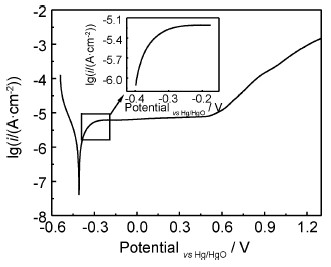
Ce68Al10Cu20Nb2大块非晶合金在1mol/L的NaOH溶液中的极化曲线如图 2所示。极化曲线上不存在活化-钝化区,这表明Ce68Al10Cu20Nb2非晶合金在NaOH溶液中会发生自钝化。极化曲线有较宽的钝化区,钝化区范围为-0.25~0.50V,维钝电流密度范围为10-5~10-6A/cm2。一般认为,金属材料的钝化区越宽,材料的耐点蚀能力越强,因此可知Ce-Al-Cu-Nb大块非晶合金在NaOH溶液中具有良好的耐点蚀能力。在阳极Tafel区没有呈现明显的直线段,如图 2中的局部放大图所示,这可能是由于该非晶合金在NaOH溶液中阳极溶解时,表面迅速形成了钝化膜,从而阻碍了合金的溶解过程[19]。将极化曲线的数据进行分析,可以得到Ce68Al10Cu20Nb2大块非晶合金在1mol/L NaOH溶液中的腐蚀电位为-0.405V,腐蚀电流密度为2.09×10-6A/cm2。
|
图2 Ce68Al10Cu20Nb2大块非晶合金在1mol/L NaOH溶液中的极化曲线 Fig.2 Polarization curves of Ce68Al10Cu20Nb2 bulk amorphous alloy in 1mol/L NaOH solution |
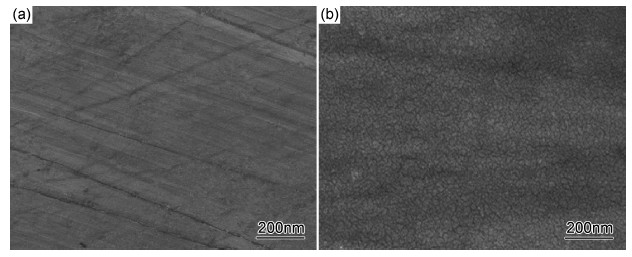
图 3(a)为Ce68Al10Cu20Nb2大块非晶合金电化学钝化前的SEM照片,在其表面只能观察到少量划痕。图 3(b)为电化学钝化后的SEM照片,在其表面可以观察到纳米颗粒状钝化膜。
|
图3 Ce68Al10Cu20Nb2大块非晶合金电化学钝化前后表面SEM照片 (a)钝化前;(b)钝化后 Fig.3 SEM images of Ce68Al10Cu20Nb2 bulk amorphous alloy surface (a)before electrochemical passivation;(b)after electrochemical passivation |
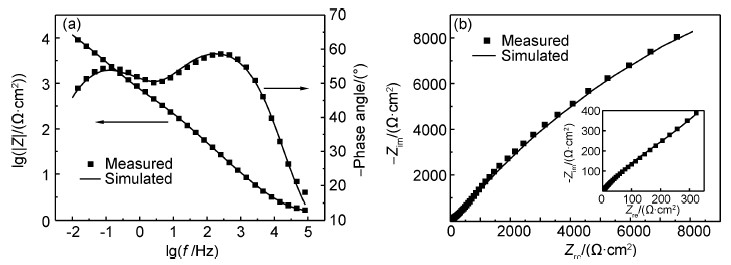
Ce68Al10Cu20Nb2大块非晶合金在1mol/L NaOH溶液中,于-0.1V下恒电位钝化30min后的EIS谱如图 4所示。从图 4(a)可以看出,Bode图中存在高频和低频相角峰,说明存在两个时间常数,这表明合金表面形成了双层结构的钝化膜。从图 4(b)中可以看出,Nyquist图由两个容抗弧组成,分别位于高频区和低频区。其中,高频容抗弧反映的是外层膜的电阻和电容,低频容抗弧则与内层膜的电阻和电容相关。
|
图4 Ce68Al10Cu20Nb2大块非晶合金在-0.1V下恒电位钝化30min后的Bode图(a)和Niquist图(b) Fig.4 Bode(a) and Niquist(b) diagrams of Ce68Al10Cu20Nb2 bulk amorphous alloy passived at -0.1V for 30min |
根据Nyquist图建立等效电路模型,如图 5所示。其中Rs为溶液电阻,Qp和Rp分别表示外层膜的常相位角元件和电阻,表征外层膜与溶液界面的反应过程。 Qb和Rb分别表示内层膜的常相位角元件和电阻,Rb越大,表明内层膜对于基体的防护作用越好。常相位角元件Q=1/Y0 (jω)n,Y0是常相位角元件Q的基本导纳,n为无量纲指数,表征Q偏离理想电容的程度[20]。EIS采用ZsimpWin软件进行参数解析,表 1为等效电路模型中的各参量经过拟合后得到的数值,等效电路图中的各参数拟合误差在4%以内,表明拟合结果和实验结果吻合良好。从图 4也可以看出,测量数据和拟合数据基本重合。
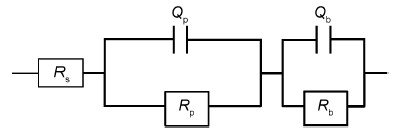
|
图5 Ce68Al10Cu20Nb2大块非晶合金在-0.1V下恒电位钝化30min后EIS的等效电路图 Fig.5 Equivalent circuits used to fit EIS of Ce68Al10Cu20Nb2 bulk amorphous alloy passived at -0.1V for 30min |
从表 1中拟合的数值可以看出,Rb和Rp分别为43000Ω·cm2和168.8Ω·cm2,Rb>>Rp,表明内层膜较为致密,外层膜较为疏松,且内层膜对非晶合金的耐腐蚀性能起决定作用。
| Rs/(Ω·cm2) | Rp/(Ω·cm2) | Qp-Y0/(Ω-1·cm-2·s-n) | np | Rb/ (Ω·cm2) | Qb-Y0/ (Ω-1·cm-2·s-n) | nb |
| 1.35 | 168.8 | 4.51×10-4 | 0.74 | 43000 | 4.6×10-4 | 0.66 |
利用 XPS对在1mol/L NaOH溶液中电化学钝化30min所形成的钝化膜的成分进行了分析。图 6为钝化膜对应刻蚀0,1,4min和10min的XPS图谱。可以看出,钝化膜外层中含有Ce元素、Nb元素和O元素,没有Al元素,Cu元素含量很少,说明Cu和Al这两种元素较之Ce和Nb,在形成钝化膜时会被优先腐蚀。从刻蚀1min以后的Cu2p (图 6(b))和Al2p(图 6(c))XPS图谱可以看出,随刻蚀时间延长,信号强度越来越大,表明越靠近外部,Cu和Al被腐蚀掉的越多。
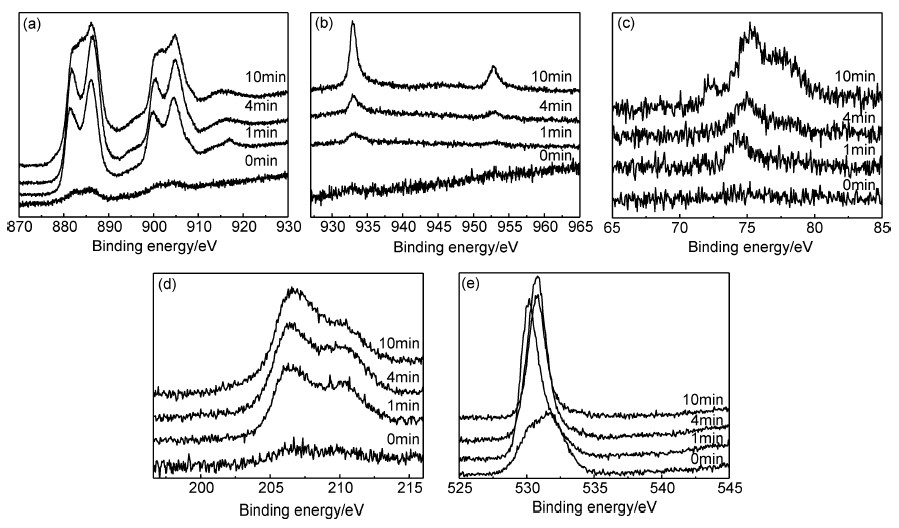
|
图6 Ce68Al10Cu20Nb2大块非晶合金表面钝化膜对应不同刻蚀时间的XPS图谱 (a)Ce3d;(b)Cu2p;(c)Al2p;(d)Nb3d;(e)O1s Fig.6 XPS spectra with different sputtering time for passive film formed on the surface of Ce68Al10Cu20Nb2 bulk amorphous alloy (a)Ce3d;(b)Cu2p;(c)Al2p;(d)Nb3d;(e)O1s |
图 7为钝化膜刻蚀10min后的XPS高分辨图谱(Ce3d,Cu2p,Al2p,Nb3d和O1s)的拟合结果。由图 7(a)Ce3d XPS图谱的分析可知,由于自旋-轨道相互作用,Ce3d的轨道分裂为两个能态,分别为Ce3d5/2 (v0,v,v′和v″)和Ce3d3/2(u0,u,u′和u″)。图谱是由4组Ce3d的自旋-轨道耦合双线组成,其中v0-u0(880.9,899.7eV)和v′-u′(886.0,904.6eV)是Ce3+的自旋-轨道耦合双线,v-u(882.5,901.1eV)和v″-u″(897.8,916.3eV)这两组是Ce4+的自旋-轨道耦合双线[21, 22, 23, 24]。对以上提到的4组峰分别积分后,可得到刻蚀10min后钝化膜中Ce3+和Ce4+的质量分数。通过计算表明,在刻蚀10min后,Ce3+ 占主导地位,约为65%。
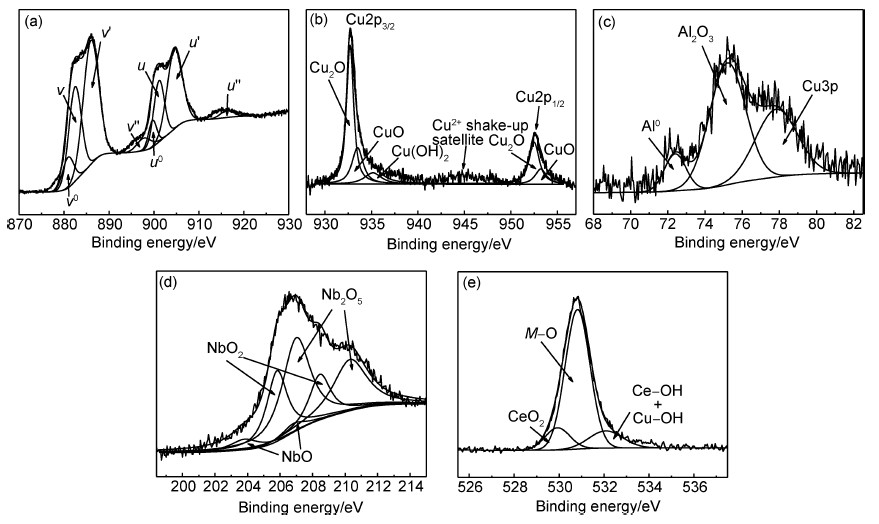
|
图7 Ce68Al10Cu20Nb2大块非晶合金表面钝化膜的XPS高分辨谱图拟合结果(刻蚀10min后)(a)Ce3d;(b)Cu2p;(c)Al2p;(d)Nb3d;(e)O1s Fig.7 Fitting curves of high resolution XPS spectra for passive film formed on the surface of Ce68Al10Cu20Nb2 bulk amorphous alloy (after sputtering for 10min) (a)Ce3d;(b)Cu2p;(c)Al2p;(d)Nb3d;(e)O1s |
由Cu2p的XPS图谱(图 7(b))可以看出,由于自旋-轨道相互作用,Cu2p的轨道分裂为两个能态,分别为Cu2p3/2和Cu2p1/2。在刻蚀10min后,Cu2p3/2包含了3个峰,其中932.7eV处的峰对应Cu+(Cu2O)和Cu0[25],933.6eV和935.1eV处的峰分别对应CuO[25]和Cu(OH)[25, 26]2中的Cu2+。939.0~945.0eV之间的峰为Cu2+的卫星伴峰[27]。Cu2p1/2包含952.4eV和953.4eV两个峰,分别对应Cu+(Cu2O)和Cu2+(CuO)[25]。以图 7(b)的分析为基础,结合图 6(b)可以看出,随着刻蚀时间的延长,CuO和Cu(OH)2的含量逐渐减少,Cu2O逐渐增加,在刻蚀10min时,Cu+比例达到66%。这是因为Cu2O在空气中不稳定,很容易氧化变成CuO,所以最外层没有Cu2O[28]。
由Al2p的XPS图谱(图 7(c))可以看出,在刻蚀10min后,Al2p分为3个峰,72.4eV是Al0[29]对应的峰,位于 75.2eV附近的峰为Al3+,对应的存在形式为Al2O[29]3,77.8eV对应的是 Cu3p,Qin等在研究Zr-Al-Cu-Ni-Pd大块非晶合金时也有此发现[30]。结合图 6(c)可以看到,在刻蚀1min后,位于 74.3eV的峰对应的是Al(OH)3中的Al3+[31]。此外,该图谱中还发现存在少量的Al2O3。以上表明,在1mol/L NaOH溶液中钝化30min后得到的钝化膜中,表层不含Al元素,内层随深度增加,Al(OH)3逐渐减少,Al2O3逐渐增加,在刻蚀到10min时,只存在 Al2O3,并出现少量Al单质。
通过对刻蚀10min后Nb3d的XPS图谱(图 7(d))进行分析,并结合不同刻蚀时间的Nb3d XPS图谱(图 6(d))可以看到,随刻蚀时间的延长,图谱并无明显变化。Nb的氧化物为NbO(203.8eV和206.8eV),NbO2(205.8eV和208.5eV)和Nb2O5(207.0eV和210.3eV)[32, 33],其中Nb2O5含量最多,NbO含量最少,在刻蚀10min时,Nb5+约为60%。
在刻蚀10min后O1s 的XPS图谱(图 7(e))中,529.8eV对应CeO2中的氧峰[34],530.9eV代表M—O键(M代表Ce,Cu,Al和Nb元素)。532.0eV对应的是Ce—OH 键[34]和Cu—OH键,此时的—OH 键含量已经很少。与之对应的钝化膜未经刻蚀时O1s的XPS图谱如图 8所示,529.8eV对应CeO2中的氧峰,530.8eV处的峰,对应的是Ce2O3,CuO及Nb的氧化物中的氧峰[32, 34],532.0eV对应的是Ce—OH键,含量最多。结合O的XPS图谱(图 6(e),图 7(e)和图 8)分析可知,随刻蚀时间的延长,氢氧化物逐渐减少,氧化物逐渐增多。

|
图8 Ce68Al10Cu20Nb2大块非晶合金表面钝化膜O1s高分辨XPS谱图的拟合结果(未刻蚀) Fig.8 Fitting curves of O1s high resolution XPS spectrum for passive film formed on the surface of Ce68Al10Cu20Nb2 bulk amorphous alloy(before sputtering) |
综上所述,结合所有XPS图谱分析可知,通过电化学钝化得到的钝化膜,外层元素分布和内层分布差别较大,外层不含Al,含有少量的Cu,在刻蚀1min后,即出现明显的Cu峰和Al峰,可知外层很薄。膜的外层由于氧含量最高,有可能形成金属氢氧化合物,如Ce(OH)3,Ce(OH)4等,从外到内,随深度增加,氢氧化物含量逐渐减少,此时膜内层主要形成金属氧化物。在内层膜中,Ce3+和Cu+的含量要比Ce4+和Cu2+多,Al和Nb则主要以Al2O3和Nb2O5居多。
3 结论(1)Ce68Al10Cu20Nb2大块非晶合金在NaOH溶液中表现出明显的自钝化行为,钝化区范围为-0.25~0.50V,维钝电流密度范围为10-5~10-6A/cm2。
(2)Ce68Al10Cu20Nb2大块非晶合金在NaOH溶液中通过电化学钝化形成内层致密、外层疏松的双层结构的钝化膜,致密内层对材料的耐腐蚀性能起决定性作用。
(3)钝化膜外层主要由Ce的氧化物和氢氧化物以及Nb的氧化物构成,此外还有少量Cu的氧化物。内层则由Ce,Cu,Al和Nb的氧化物及氢氧化物构成,由外到内,随深度增加,氢氧化物逐渐减少,氧化物最终占据绝大部分。
| [1] | 汪卫华. 金属玻璃研究简史[J]. 物理,2011, 40 (11) : 701 –709. WANG Wei-hua. A brief history of metallic glasses[J]. Physics,2011, 40 (11) : 701 –709. |
| [2] | 胡壮麒, 张海峰. 块状非晶合金及其复合材料研究进展[J]. 金属学报,2010, 46 (11) : 1391 –1421. HU Zhuang-qi, ZHANG Hai-feng. Recent progress in the area of bulk amorphous alloy and composites[J]. Acta Metallurgica Sinica,2010, 46 (11) : 1391 –1421. |
| [3] | PARK E S, KIM D H. Design of bulk metallic glasses with high glass forming ability and enhancement of plasticity in metallic glass matrix composites: a review[J]. Metals and Materials International,2005, 11 (1) : 19 –27. |
| [4] | ZHANG B, ZHAO D Q, PAN M X, et al. Amorphous metallic plastic[J]. Physical Review Letters,2005, 94 (20) : 1 –4. |
| [5] | ZHANG B, WANG R J, ZHAO D Q, et al. Properties of Ce-based bulk metallic glass-forming alloys[J]. Physical Review B,2004, 70 (22) : 1 –7. |
| [6] | ZHANG T, LI R, PANG S. Effect of similar elements on improving glass-forming ability of La-Ce-based alloys[J]. Journal of Alloys and Compounds,2009, 483 (1-2) : 60 –63. |
| [7] | LI R, PANG S J, MEN H, et al. Formation and mechanical properties of (Ce-La-Pr-Nd)-Co-Al bulk glassy alloys with superior glass-forming ability[J]. Scripta Materialia,2006, 54 (6) : 1123 –1126. |
| [8] | BAI Y Y, GENG Y L, JIANG C M, et al. β relaxation and its composition dependence in Ce-based bulk metallic glasses[J]. Journal of Non-Crystalline Solids,2014, 390 : 1 –4. |
| [9] | FORNELL J, SURIÑACH S, BARÓ M D, et al. Unconventional elastic properties, deformation behavior and fracture characteristics of newly developed rare earth bulk metallic glasses[J]. Intermetallics,2009, 17 (12) : 1090 –1097. |
| [10] | WEI B C, ZHANG T H, ZHANG L C, et al. Plastic deformation in Ce-based bulk metallic glasses during depth-sensing indentation[J]. Materials Science and Engineering: A,2007, 449-451 : 962 –965. |
| [11] | ZHANG L C, WEI B C, XING D M, et al. The characterization of plastic deformation in Ce-based bulk metallic glasses[J]. Intermetallics,2007, 15 (5-6) : 791 –795. |
| [12] | XU B C, XUE R J, ZHANG B. Superior glass-forming ability and its correlation with density in Ce-Ga-Cu ternary bulk metallic glasses[J]. Intermetallics,2013, 32 : 1 –5. |
| [13] | ZHOU Y, ZHAO Y, QU B Y, et al. Remarkable effect of Ce base element purity upon glass forming ability in Ce-Ga-Cu bulk metallic glasses[J]. Intermetallics,2014, 56 : 56 –62. |
| [14] | QIAO J C, PELLETIER J M. Thermal stability of (Ce0.72Cu0.28)90-xAl10Fex(x=0, 5 or 10) bulk metallic glasses[J]. Physica Status Solidi (c),2011, 8 (11-12) : 3074 –3077. |
| [15] | YU P, CHAN K C, CHEN W, et al. Low-temperature mechanical properties of Ce[J]. Philosophical Magazine Letters,2011, 91 (1) : 70 –77. |
| [16] | 胡侨, 张敏, 李海飞, 等. Ti-Zr-Cu-Co-Sn-Si块体非晶合金的形成及生物腐蚀行为和力学性能[J]. 材料工程,2014 (6) : 18 –21. HU Qiao, ZHANG Min, LI Hai-fei, et al. Formation, bio-corrosion behavior and mechanical properties of Ti-Zr-Cu-Co-Sn-Si bulk metallic glasses[J]. Journal of Materials Engineering,2014 (6) : 18 –21. |
| [17] | 邱春龙, 黄璐, 卢旭阳, 等. Ni-Ti(-Zr)-P非晶合金的热稳定性及腐蚀行为[J]. 稀有金属材料与工程,2013, 42 (5) : 975 –978. QIU Chun-long, HUANG Lu, LU Xu-yang, et al. Thermal stability and corrosion behavior of Ni-Ti(-Zr)-P glassy alloys[J]. Rare Metal Materials and Engineering,2013, 42 (5) : 975 –978. |
| [18] | BIAN Z, INOUE A. New Ce-Cu-Al-Zn bulk metallic glasses with high oxidation resistance[J]. Materials Transactions,2006, 47 (10) : 2599 –2602. |
| [19] | GEBERT A, MUMMERT K, ECKERT J, et al. Electrochemical investigations on the bulk glass forming Zr55Cu30Al10Ni5 alloy[J]. Materials and Corrosion,1997, 48 (5) : 293 –297. |
| [20] | WU H, WANG Y, ZHONG Q, et al. The semi-conductor property and corrosion resistance of passive film on electroplated Ni and Cu-Ni alloys[J]. Journal of Electroanalytical Chemistry,2011, 663 (2) : 59 –66. |
| [21] | 纪红, 许越, 周德瑞, 等. LY12铝合金表面铈纳米膜的制备及显微组织特征[J]. 航空材料学报,2003, 23 (1) : 21 –23. JI Hong, XU Yue, ZHOU De-rui, et al. Process and microstructure characters of ceria nanocrystalline film on aluminium alloy LY12[J]. Journal of Aeronautical Materials,2003, 23 (1) : 21 –23. |
| [22] | MONTEMOR M F, SIMÕES A M, FERREIRA M G S, et al. Composition and corrosion resistance of cerium conversion films on the AZ31 magnesium alloy and its relation to the salt anion[J]. Applied Surface Science,2008, 254 (6) : 1806 –1814. |
| [23] | 康俊龙, 姚兰芳, 杨松林, 等. Ce掺杂TiO2纳米复合薄膜的制备及光催化活性[J]. 人工晶体学报,2013, 42 (4) : 671 –676. KANG Jun-long, YAO Lan-fang, YANG Song-lin, et al. Preparation and photocatalytic activity of Ce-doped TiO2 composite nanometer films[J]. Journal of Synthetic Crystals,2013, 42 (4) : 671 –676. |
| [24] | LARACHI F, PIERRE J, ADNOT A, et al. Ce 3d XPS study of composite CexMn1-xO2-y wet oxidation catalysts[J]. Applied Surface Science,2002, 195 (1-4) : 236 –250. |
| [25] | TAN C W, DAUD A R, YARMO M A. Corrosion study at Cu-Al interface in microelectronics packaging[J]. Applied Surface Science,2002, 191 (1-4) : 67 –73. |
| [26] | PROCACCINI R, SCHREINER W H, VAZQUEZ M, et al. Surface study of films formed on copper and brass at open circuit potential[J]. Applied Surface Science,2013, 268 : 171 –178. |
| [27] | MARQUES M T, FERRARIA A M, CORREIA J B, et al. XRD, XPS and SEM characterisation of Cu-NbC nanocomposite produced by mechanical alloying[J]. Materials Chemistry and Physics,2008, 109 (1) : 174 –180. |
| [28] | KUNZE J, MAURICE V, KLEIN L H, et al. In situ STM study of the duplex passive films formed on Cu(111) and Cu(001) in 0.1 M NaOH[J]. Corrosion Science,2004, 46 (1) : 245 –264. |
| [29] | BRAJPURIYA R, SHRIPATHI T. Investigation of Fe/Al interface as a function of annealing temperature using XPS[J]. Applied Surface Science,2009, 255 (12) : 6149 –6154. |
| [30] | QIN F X, ZHANG H F, CHEN P, et al. Corrosion behavior of bulk amorphous Zr55Al10Cu30Ni5-xPdx alloys[J]. Materials Letters,2004, 58 (7-8) : 1246 –1250. |
| [31] | WANG X M, ZHU L Q, HE X, et al. Effect of cerium additive on aluminum-based chemical conversion coating on AZ91D magnesium alloy[J]. Applied Surface Science,2013, 280 : 467 –473. |
| [32] | MILOŠEV I, KOSEC T, STREHBLOW H H. XPS and EIS study of the passive film formed on orthopaedic Ti-6Al-7Nb alloy in Hank's physiological solution[J]. Electrochimica Acta,2008, 53 (9) : 3547 –3558. |
| [33] | HALBRITTER J. On the oxidation and on the superconductivity of niobium[J]. Applied Physics A,1987, 43 (1) : 1 –28. |
| [34] | YU X, LI G. XPS study of cerium conversion coating on the anodized 2024 aluminum alloy[J]. Journal of Alloys and Compounds,2004, 364 (1-2) : 193 –198. |
 2016, Vol. 44
2016, Vol. 44


