文章信息
- 王红星, 谈淑咏, 柳秉毅, 沈彤. 2015.
- WANG Hong-xing, TAN Shu-yong, LIU Bing-yi, SHEN Tong. 2015.
- 纳米SiC浓度对Ni/纳米MoS2基复合镀层结构和耐磨性能的影响
- Effect of Nano-SiC Content on Microstructure and Wear Resistance of Ni/Nano-MoS2 Based Composite Coating
- 材料工程, 43(10): 60-65
- Journal of Materials Engineering, 43(10): 60-65.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2015.10.010
-
文章历史
- 收稿日期:2014-09-04
- 修订日期:2015-04-20
复合电镀是通过金属电沉积的方法,将一种或几种不溶性固体颗粒(Al2O3,SiC,TiC等)均匀地夹杂到金属基质中,形成具有特殊性能的镀层。随着电镀技术和纳米制备技术的发展,纳米复合镀层已成为国内外许多学者关注的热点。采用复合电镀技术,以纳米颗粒替代微米颗粒,可制备比微米复合镀层各项性能更为优异的纳米复合镀层。
目前,在镍基复合镀层中,加入不溶性颗粒如Al2O[1, 2, 3]3,BN[4],SiC[5, 6, 7],Si3N4 [8],TiC[9]等高硬度的颗粒形成具有高硬度和高耐磨性的复合镀层;另外,加入具有自润滑性的颗粒如PTFE[10],MoS[11, 12]2和WS[13]2等形成具有减摩性能的复合镀层。而在镀液中同时加入这两种不同性质的颗粒,可得到既有较好耐磨性又有良好自润滑性的复合镀层。Wu等[14]采用化学镀制备了Ni-P-SiC-PTFE复合镀层,研究表明,Ni-P-SiC-PTFE复合镀层的减摩性能优于Ni-P-SiC,而耐磨性能又高于Ni-P-PTFE镀层,表现出良好的减摩-耐磨综合性能;Huang等[15]采用化学镀的方法,分别制备了纯Ni镀层,Ni-SiC,Ni-PTFE和Ni-SiC-PTFE复合镀层,研究表明,Ni-SiC-PTFE复合镀层综合了Ni-SiC和Ni-PTFE的优点,滑动磨损量为纯镍镀层的1/7;Wang等[16]采用微弧氧化技术,在铝合金表面制备了Al2O3/PTFE复合镀层,与单一的Al2O3层相比,复合镀层具有更低的磨损量、低而稳定摩擦因数;Huang等[17]首先对MoS2颗粒包覆Al2O3,然后采用复合电镀的方法,在镀镍溶液中制备Ni/MoS2/Al2O3复合镀层,显微硬度和耐磨性能明显高于单一的Ni/MoS2镀层。王兰等[18]采用化学镀制备了Ni-P-SiC-MoS2复合镀层,镀层的硬度低于Ni-P-SiC而高于Ni-P-MoS2镀层,摩擦磨损性能都优于Ni-P-SiC 和Ni-P-MoS2镀层。丁元柱等[19]采用电刷镀技术,制备了具有良好的耐磨减摩纳米SiC-MoS2/Ni复合刷镀层。
复合电沉积技术具有工艺简单、成本低、可以在常温下操作、不影响主体材料内部性质等优点,在耐磨减摩复合材料的研究与开发中占有重要的地位。采用双脉冲电源共沉积制备Ni-MoS2-SiC复合镀层的研究鲜见报道。本工作在前期研究[20]的基础上,主要研究了Ni/MoS2复合镀层基础上,镀液中添加纳米SiC颗粒,研究复合镀液中纳米SiC颗粒浓度对Ni/MoS2复合镀层组织结构及摩擦性能的影响,这方面的研究具有一定的理论意义与实用价值。
1 实验方法以纯镍板为阳极,99.99%的紫铜块切割成尺寸为15mm×10mm×2mm的试样为阴极,采用双脉冲电源,在纯铜上沉积Ni-MoS2基纳米复合镀层。电镀液组成为:硫酸镍(NiSO4·6H2O)300g/L、氯化镍(NiC12·6H2O)30g/L、硼酸(H3BO3)30g/L和适量的表面活性剂十二烷基硫酸钠。复合镀液中纳米MoS2颗粒的粒径为40nm,加入浓度为2.5g/L,纳米SiC的粒径为40nm,镀液中加入量为0.5,1.0g/L和1.5g/L。电沉积工艺参数及条件:正向电流密度为2A/dm2,反向电流密度为0.2A/dm2,脉冲频率为1kHz,脉冲占空比为0.5,镀液温度采用水浴控制在30℃,镀液采用机械搅拌,转速为200r/min,电镀时间为10h。电镀前,纳米颗粒加入预先配制好的镀液中,机械搅拌0.5h,再超声波震荡15min后,加入镀液并机械搅拌15min后复合镀。
采用WTM-2E型可控气氛微型摩擦磨损仪。测试条件:Si3N4球为对磨材料,直径φ4mm;载荷200g,转速为250r/min,环境温度为室温,磨痕半径为4mm。采用FM-700型显微硬度机测试,所加载荷为100g,加载时间为15s,在试样截面5个不同部位测试硬度,取平均值。
采用JSM-6380LV扫描电镜(SEM)配能谱分析仪(EDS)分析渗层表面及磨损表面的微观形貌;采用 Bruker D8 Advance 型转靶 X 射线衍射仪(XRD)分析镀层结构,旋转靶为铜靶,采用CuKα射线,管电流40mA,管电压40kV,步长0.02°,扫描速率为5.0(°)/min。
2 实验结果与分析 2.1 镀液中纳米SiC浓度对镀层微观形貌和结构的影响图 1为镀液中添加不同浓度纳米SiC颗粒后,Ni/MoS2基复合镀层的微观形貌。其中,图 1(a)为未添加纳米SiC颗粒,图 1(b)~(d)为镀液中分别添加0.5,1.0g/L和1.5g/L纳米SiC颗粒。镀液中添加纳米SiC颗粒后,对复合镀层的微观形貌有明显的影响。从图 1中微观形貌看出,镀液中未添加纳米SiC时,Ni-MoS2复合镀层疏松;镀液中添加0.5g/L SiC后,镀层的致密度没有明显改善;随着镀液中纳米SiC颗粒的浓度增加到1.0g/L SiC时,复合镀层的致密性提高,如图 1(c)所示;继续增加镀液中SiC的浓度到1.5g/L时,复合镀层的微观形貌无明显变化。这是由于MoS2 是导电性微粒,当纳米颗粒被吸附到镀层上,镍离子不但沉积在镀镍层表面,还覆盖在纳米颗粒上并突出镀层表面,镍离子在这些突点处沉积的速率比较快;突点覆盖有镍金属层也更易于捕获镀液中的MoS2,并形成了树枝状组织成长[21]。镀液中添加非导体的纳米SiC颗粒后,SiC 颗粒本身导电性能差,不会直接作为镍结晶形核的有利场所而参与电沉积过程,在吸附了大量阳离子的纳米碳化硅颗粒在阴极表面黏附,颗粒可以通过它们吸附离子层促进离子输送到阴极表面,对镍结晶产生了扰动作用,改变了MoS2在镀液中单独存在时的生长方式;另一方面,由于纳米颗粒量多,碰撞几率加大,出现团聚体,镀层中SiC 粒子增多,形成表面堆积,复合镀层表面呈“菜花”状。经EDS分析,镀液中分别添加0.5,1.0g/L和1.5g/L 纳米SiC颗粒后,镀层中Si的原子分数分别为1.70%,2.34%和3.27%。
 |
图 1 镀液中不同SiC浓度时复合镀层的微观形貌 (a)未添加;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L Fig. 1 SEM images of the surface of composite coatings with different SiC content in the solution (a)without addition SiC;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L |
图 2为镀液中添加不同浓度纳米SiC颗粒后,Ni-SiC复合镀层截面微观形貌。由图 2看出,本实验镀液中纳米SiC颗粒添加量在0.5~1.5g/L范围内,纳米SiC颗粒较均匀分散在镀层内,且含量随镀液中颗粒浓度的增加而增加。
图 3为镀液中不同SiC浓度时复合镀层XRD图谱。从图 3看出,镀液中添加 0.5g/L纳米SiC后,衍射图谱中没有出现SiC衍射峰,由于复合镀层内纳米SiC颗粒的复合量较少,复合镀层主要有Ni和MoS2组成。随镀液中纳米SiC颗粒添加量的增加,镀液中纳米SiC颗粒添加量超过1.0 g/L时,XRD图谱中出现了SiC的特征峰。这是由于镀液中添加纳米SiC颗粒后,SiC颗粒占据了阴极表面局部位置,与镍共沉积到复合镀层中,形成了含纳米MoS2和SiC组成的复合镀层。
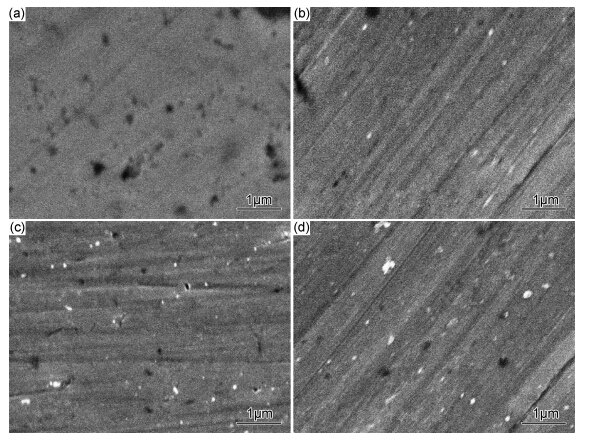 |
图 2 复合镀层的截面微观形貌 (a)未添加;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L Fig. 2 SEM images of the cross-section of composite coatings with different nano-SiC content in the solution (a)without addition SiC;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L |
 |
图 3 镀液中不同SiC浓度时复合镀层XRD图谱 (a)未添加;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L Fig. 3 XRD patterns of the composite coatings with different SiC content in the solution (a)without addition SiC;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L |
图 4为镀液中纳米SiC颗粒的加入量与复合镀层显微硬度的关系。从图 4看出,随加入量的增加,镀层的显微硬度逐渐增大。镀液中未添加SiC时,复合镀层的显微硬度为365HV。镀液中纳米SiC颗粒加入量为1.5g/L时,显微硬度为505HV。在纳米SiC颗粒添加前,Ni-MoS2复合镀层的显微硬度提高,归因于纳米MoS2 在镀层内的弥散强化作用所致;镀液添加纳米SiC颗粒后,复合镀层的显微硬度主要由镀层内纳米SiC复合量决定,SiC是硬质材料,当共沉积到镀层中,在镀层中起弥散强化和颗粒强化作用,复合量越大,镀层硬度越高。
 |
图 4 镀液中 SiC浓度对复合镀层硬度的影响 Fig. 4 The effect of SiC particles content in the solution on hardness of coatings |
图 5是在不同添加物浓度SiC的影响下镀层的摩擦因数与时间关系的变化曲线图。从图 5可以看出,当镀液中不添加SiC时,镀层的摩擦因数波动小,滑动5min后,摩擦因数一直维持在0.57左右;镀液中添加0.5g/L SiC时,镀层的摩擦因数减小,在30min滑动时间内,基本维持在0.45左右;镀液中添加1.0g/L SiC时,滑动5min后,摩擦因数在0.39~0.42之间波动;镀液中添加1.5g/L SiC时,摩擦因数波动较大,摩擦因数维持在0.25~0.29。复合镀层的摩擦因数随镀液中纳米SiC增加而降低,这主要归因于镀层内硬质相SiC颗粒复合量增加,与复合镀层的显微硬度结果相吻合。
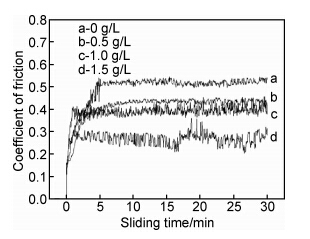 |
图 5 摩擦因数随时间变化的曲线 Fig. 5 Relationship between coefficient and sliding time |
图 6为镀液中添加不同浓度SiC颗粒的复合镀层表面磨痕微观形貌。由图 6可知,Ni/MoS2 复合镀层的磨损犁沟较宽,而且还观察到磨痕表面有材料转移的痕迹,复合镀层由磨粒磨损为主的黏着-磨粒磨损混合机制组成,如图 6(a)所示;镀液中添加0.5g/LSiC后,复合镀层表面的磨损机制与未添加纳米SiC时相类似;当镀液中添加SiC颗粒浓度超过1.0g/L后,由图 6(c)中可明显观察硬质颗粒造成的变形和摩擦痕迹,表面分布着平行于滑动方向的犁沟,呈典型的磨粒磨损机制;镀液中添加1.5g/L SiC后,复合镀层的磨痕表面明显与其他条件下制备的镀层不同,犁沟方向与滑动方向一致但不连续,呈网络状,如图 6(d)所示。这是由于复合镀层内的MoS2和SiC分别为软质、硬质材料,在抵抗磨损的过程中,起着不同的作用,镀层中的MoS2在磨损过程中与对磨件摩擦时,能在接触表面之间形成一层MoS2,固体润滑膜,起到润滑作用,降低镀层的摩擦因数,SiC第二相固体微粒嵌入基质金属后,高硬度能有效地提高材料抗黏着和抗磨粒磨损的能力,会阻碍其位错的移动和晶格的畸变,对镀层起到弥散强化的作用,复合量越大,复合镀层抵抗塑性变形的能力越强,因此镀层具有较低的摩擦因数和较高的硬度。
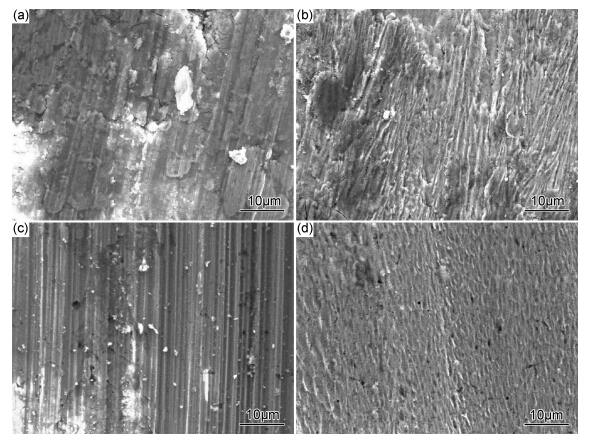 |
图 6 镀液中添加不同浓度SiC的复合镀层磨痕形貌 (a)未添加;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L Fig. 6 SEM morphology of the wear tracks of the composites coatings deposited with different content of nano SiC particles in electrolyte (a)without addition SiC;(b)0.5g/L;(c)1.0g/L;(d)1.5g/L |
(1)镀液中添加纳米后,复合镀层的微观形貌发生了改变,随镀液中纳米SiC浓度的增加,镀层表面致密度提高;复合镀层的组织主要由Ni+MoS2+SiC组成。
(2)镀液中添加纳米SiC后,复合镀层硬度明显提高,并随镀液中纳米SiC浓度的增加而增加,当镀液中纳米SiC添加量为1.5g/L时,复合镀层显微硬度达到最大,为505HV,摩擦因数最小,为0.28,分别为纯Ni/MoS2的1.6倍和1/2。复合镀层的磨损机制以磨料磨损为主。
| [1] | ALIREAZIE S, MONIRVAGHEFI S M, SALEHI M, et al. Wear behavior of Ni-P and Ni-P-Al2O3 electroless coatings[J]. Wear,2007,262:978-985. |
| [2] | CHEN L, WANG L P, ZENG Z X, et al. Influence of pulse frequency on the microstructure and wear resistance of electrodeposited Ni-Al2O3 composite coatings [J]. Surface and Coatings Technology 2006,201 (3-4):599-605. |
| [3] | FENG Q Y, LI T J, YUE H Y, et al. Preparation and characterization of nickel nano-Al2O3 composite coatings by sediment co-deposition [J]. Applied Surface Science, 2008, 254 (8):2262-2268. |
| [4] | POMPEI E, MAGAGNIN L, LECIS N, et al. Electrodeposition of nickel-BN composite coatings[J]. Electrochimica Acta 2009, 54 (9):2571-2574. |
| [5] | LOW C T J,WILLS R G A,WALSH F C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit [J]. Surface and Coatings Technology, 2006, 201(1-2):371-383. |
| [6] | HACHEMI B T, ABDELOUAHED C, SAÂD R. Microhardness and corrosion behavior of Ni-SiC electrodeposited coatings in presence of organic additives [J]. Surface and Coatings Technology, 2011,205(Suppl 2): 161-164. |
| [7] | GVL H, UYSAL F K M, ASLAN S, et al. Effect of particle concentration on the structure and tribological properties of submicron particle SiC reinforced Ni metal matrix composite (MMC) coatings produced by electrodeposition [J].Applied Surface Science, 2012,258 (10):4260-4267. |
| [8] | BALARAJU J N, EZHIL S V, RAJAM K S. Electrochemical behavior of low phosphorus electroless Ni-P-Si3N4 composite coatings [J].Materials Chemistry and Physics,2010, 120(2-3): 546-551. |
| [9] | KARBASI M, YAZDIAN N, VAHIDIAN A. Development of electro-co-deposited Ni-TiC nano-particle reinforced nanocomposite coatings [J].Surface and Coatings Technology, 2012, 207:587-593. |
| [10] | ZHAO Q, LIU Y, ABEL E W. Effect of Cu content in electroless Ni-Cu-P-PTFE composite coatings on their anti-corrosion properties [J]. Materials Chemistry and Physics, 2004, 8 (7): 332-335. |
| [11] | LIEW K W, CHIA S Y, KOK C K, et al. Evaluation on tribological design coatings of Al2O3, Ni-P-PTFE and MoS2 on aluminium alloy 7075 under oil lubrication [J]. Materials and Design, 2013, 48:77-84. |
| [12] | CARDINAL M F, CASTRO P A, BAXI J, et al. Characterization and frictional behavior of nanostructured Ni-W-MoS2 composite coatings [J]. Surface and Coatings Technology, 2009,204 (1-2): 85-90. |
| [13] | GARCIA L E,GARCIA U I,DIEZ J A,et al. Codeposition of inorganic fullerene-like WS2 nanoparticles in an electrodeposited nickel matrix under the influence of ultrasonic agitation [J].Electrochimica Acta, 2013,114:859-867. |
| [14] | WU Y T, LIU H Z, SHEN B, et al. The friction and wear of electroless Ni-P matrix with PTFE and/or SiC particles composite [J]. Tribology International 2006, 39 (6): 553-559. |
| [15] | HYANG Y S, ZENG X T, ANNERGREN I, et al. Development of electroless NiP-PTFE-SiC composite coating [J]. Surface and Coatings Technology, 2003,167 (2-3): 207-211. |
| [16] | WANG Z J, WU L N, QI Y L, et al. Self-lubricating Al2O3/PTFE composite coating formation on surface of aluminium alloy [J]. Surface and Coatings Technology, 2010,204(20): 3315-3318. |
| [17] | HUANG Z J, XIONG D S. MoS2 coated with Al2O3 for Ni- MoS2/Al2O3 composite coatings by pulse electrodepositon [J]. Surface and Coatings Technology, 2008, 202 (14):3208-3214. |
| [18] | 王兰,邵红红,陈康敏,等.Ni-P-SiC-MoS2复合镀层结构与性能研究[J].腐蚀与防护,2006,27(6):334-337. WANG Lan, SHAO Hong-hong, CHEN Kang-min, et al.Structure and performance of electroless composite Ni-P-SiC-MoS2 plating [J].Corrosion and Protection, 2006,27(6):334-337. |
| [19] | 丁元柱,王研.纳米SiC-MoS2/Ni基复合电刷镀层组织与耐磨性能[J]. 材料开发与应用,2010,25(6):44-47. DING Yuan-zhu, WANG Yan.Microstructure and wear resistance of nano-SiC-MoS2/Ni electro-brush plating composite coating[J]. Development and Application of Materials, 2010,25(6):44-47. |
| [20] | 王红星,杨少锋,柳秉毅,等.料浆包渗温度对渗Si层组织结构和性能的影响[J].材料工程,2013,(2):69-73. WANG Hong-xing, YANG Shao-feng, LIU Bing-yi, et al.Effect of packing temperature on micro structure and properties of siliconized coating [J]. Journal of Materials Engineering,2013,(2):69-73. |
| [21] | HUANG Z J, XIONG D S. MoS2 coated with Al2O3 for Ni-MoS2 /Al2O3 composite coatings by pulse electro-deposition[J]. Surface and Coatings Technology, 2008,202(14):3208-3214. |




