文章信息
- 喇培清, 欧玉静, 韩少博, 卢学峰, 魏玉鹏. 2015.
- LA Pei-qing, OU Yu-jing, HAN Shao-bo, LU Xue-feng, WEI Yu-peng. 2015.
- NaCl加入量对自蔓延高温燃烧合成法大规模制备的超细二硼化钛粉体性能的影响
- Effect of NaCl Addition on Properties of Submicron Titanium Diboride Powders Prepared by Self-propagating High-temperature Synthesis on a Large Scale
- 材料工程, 43(7): 14-20
- Journal of Materials Engineering, 43(7): 14-20.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2015.07.003
-
文章历史
- 收稿日期:2014-05-15
- 修订日期:2014-12-15
2. 兰州理工大学 石油化工学院, 兰州 730050
2. School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, China
TiB2 has been widely used as conductive ceramic materials,ceramic cutting tools and molds,composite ceramics,electrode materials and cathodes for electro-chemical processing of aluminum in Hall-Heroults process,heating ceramic materials and metal materials enhancer because of its unique and fascinating properties such as high melting temperature,hardness,elastic modulus,good electro-conductibility and thermal diffusivity,and excellent refractory properties and chemical inertness[1, 2, 3, 4, 5, 6, 7]. At present,TiB2 has been produced by the reduction of TiO2 and B2O3 with carbon and active metal,solid state reduction of TiCl4 and the self-propagating high-temperature synthesis method[8, 9, 10, 11, 12]. From the viewpoint of reducing costs,combustion synthesis,also known as self-propagating high-temperature synthesis (SHS),widely used to prepare a great diversity of ceramic powders[13],is a preferable technique owing to its lower energy consumption and simplified equipment.
However,due to the high temperature in the process of the self-propagating high-temperature synthesis which leads to the rapid particle growth rate,it is very difficult to obtain pure and ultrafine-particle TiB2 powder. NaCl addition as diluent to prepare TiB2 powder with high pure and ultrafine-particle size has been proved an efficient way[14, 15]. The introduction of soluble salt into mixture precursor can effectively prevent particles aggregation and forming submicron TiB2 and greatly improve the purity[16]. However,the effect of NaCl addition on morphology,size and phase of submicron TiB2 powders prepared by self-propagating high-temperature synthesis on a large scale has never been reported,to our knowledge.
Thus,we report the combustion synthesis of submicron TiB2 powder on a large scale combining with the employment of soluble salt. Effect of NaCl addition on the microstructure,average particle size and phases of the final products are investigated. Moreover,according to the experimental results and characterizations,the reaction procedure of combustion synthesis and the formation mechanism of TiB2 are discussed.
1 Experiment 1.1 Synthesis of submicron TiB2The raw materials used in this paper were commercial grade B2O3 (>99.0%,mass fraction,same as below,Liaoning Pengda Science and Technology Ltd.,Yingkou city),TiO2 (>99.5%,Shanghai Jianghutaibai Chemical Products Co.,Shanghai city),Mg (99.5%,Kunshan Fuerbang New Material Technology Co.,Ltd.,Kunshan city) and NaCl powders (99.0%,Nanjing Dongde Chemical Technology Co.,Ltd.,Nanjing city). The reaction follows the formula (1). Considering the high combustion temperature and low-melting-point Mg volatile loss[17],Mg excess 5% and k of NaCl increases by 0.5 increment until the system is incapable of self-propagating reaction. The total material quantity is 2000g. Reactant powders were mixed in planetary ball mill with the ball-to-powder mass ratio of 2 ∶1 for 8h. The obtained mixture was uniaxially pressed to form cylindrical pellets (25.4cm in diameter and about 2.5cm high) of preform at 15MPa. Original sample was then loaded into home-made reaction chamber of SHS reactor. The reaction chamber was evacuated and filled with argon gas at the pressure of 0.5MPa. The igniting tablet was placed in the copper crucible of the reaction chamber,and the sample was smoothly on the tablet. Autoclave was sealed and then heated,while argon was filled with 0.5MPa,released to eliminate the autoclave air after 10 min. Until the internal temperature was heated to about 180℃,argon was filled with 2 MPa. When the reaction chamber was about 260℃,the internal temperature and pressure increased sharply,and the self-propagating reaction started and completed within a few minutes. Reactants turned into block product containing TiB2. After the block product was grinded into powders,NaCl was washed away with distilled water and byproduct MgO was leached out from the powder with dilute HCl ( 9.6mol·L-1 ) excess of 50% following reaction (2). Leaching time lasted 72h,and the leaching liquid was stirred 1 time every 4h. TiB2 was separated from leaching solution by filtration,washed 5 times with distilled water to remove the residue of HCl. Then the TiB2 powders were put in a vacuum oven and dried for 8 hours under 100℃.
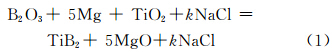


The phase analysis of the powder samples was investigated by X-ray diffraction (XRD,D/max-2400). The morphology and identification of TiB2 powder was examined by scanning electron microscope (SEM,JSM-6700) equipped with an energy dispersive spectrometer (EDS). The content of Mg and O impurities in the leached TiB2 powders was determined by atomic absorption spectrum(CAAM-2001,Haiguang GGX-6).
2 Results and discussions 2.1 Effect of NaCl addition on morphology of TiB2 powders before leachingSamples were prepared with different ratio (k=0.5,1.0,1.5,2.0mol) of NaCl. Reactant mixture could be ignited under these k values. However,when k increased to 2.25,the combustion synthesis reaction system could not occur. Merzhanov,etc[18]. proposed empirical criterion: only when the adiabatic temperature Tad ≥1800K,can the SHS reaction be self-sustained,otherwise it needs supplementary energy. When k increases to 2.25,the combustion synthesis reaction system cannot occur,and thus it is estimated adiabatic temperature drops below 1800K.
The product morphology with different amount of NaCl is shown in Fig.1. As shown in Fig.1,weak agglomeration of spherical particles with a large size of about 200-500nm can be observed. Simultaneously,melting sintering phenomenon of particles can also be observed and small particles adhere to the surface of large particles. For the sample of k=2.0,small particles agglomerates into a flocculent. In the process of combustion synthesis,a certain amount of TiB2 particles was generated and saturated in the NaCl melt. As the system temperature was reduced,it was difficult for the precipitated particles in a solid environment to grow up continuously,which resulted in a smaller size of TiB2. At the same time,small particles agglomerated into a flocculent,which might contribute to the generation of MgO along with the formation of the products. When the temperature decreased,the resulting sample was coated with MgO and NaCl. Product and MgO were in the environment of NaCl melt. With the processing of the reaction,the system temperature decreased,and the final desired product particles were coated with MgO and NaCl,or MgO and NaCl existed between TiB2 particles. This resulted in the occurrence of sintering phenomenon and the generation of large-size aggregates. For the sample of k = 1.5,a rod-like morphology is observed,which contribute to the presence of large quantity of NaCl melt in the combustion synthesis inducing the liquid phase sintering on the melt surface for large spherical particles precipitated by supersaturation. EDX analysis results show that TiB2 purity after leaching is above 98%. It is consistent with the results of atomic absorption spectrum.
 |
Fig.1 SEM photos of combustion synthesis product with different amount of diluent (before leaching) (a)k=0.5;(b)k=1.0;(c)k=1.5;(d)k=2.0 | |
Fig.2 shows SEM micrographs of TiB2 obtained from combustion synthesis with different amount of NaCl after leaching. Compared to large size aggregates composed of a large number of small particles before leaching,weak aggregates formed by submicron particles with the size of 0.2μm can be observed after leaching. Melting sintering phenomenon between the particles is also seen. The degree of sintering decreases with the increase of diluent. The particles show a state of loose agglomeration without significant sintering phenomenon and the size of aggregates drops dramatically. Submicron particles show a spherical or ellipsoidal shape with smooth surface and smaller gap between particles. The removal of surface layers of NaCl and MgO on the particles results in the decrease of grain size. With the increase of NaCl amount,which leads to the drop of combustion temperature in system,the lower particle formation temperature and weak melting sintering degree are obtained.
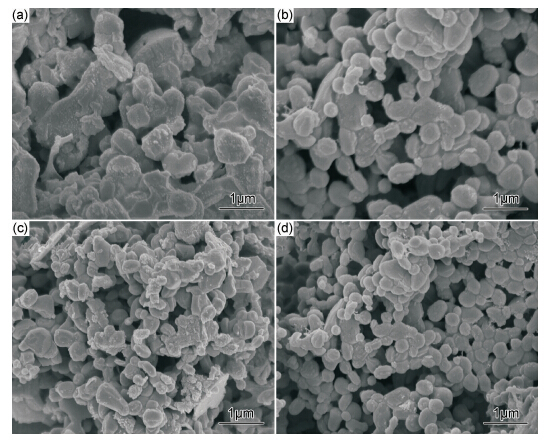 |
Fig.2 SEM photos of combustion synthesis product with different amount of diluent (after leaching) (a)k=0.5;(b)k=1.0;(c)k=1.5;(d)k=2.0 | |
The statistics of submicron particle size distribution is shown in Fig.3. The particle size follows normal distribution,and the range of distribution becomes narrow gradually with the increase of the diluent. At k=0.5,the particle size is in the range of 350 to 750nm. When the value of k increases to 2.0,the particle size decreases to 100-450nm. The increase of diluent leads to the decrease of the average particle size,as shown in Fig.4. At k = 0.5,the average particle size is 496 nm and decreases to 268nm when the value of k increases to 2.0,therefore average particle size of the products decreases with the increase of diluent amount.
 |
Fig.3 Particle size of combustion synthesis product with different addition of diluent (after leaching) (a)k=0.5;(b)k=1.0;(c)k=1.5;(d)k=2.0 | |
 | Fig.4 Dependence of mean particle size on NaCl addition (after leaching) | |
Fig.5 shows the XRD patterns of the products with different addition of NaCl. As seen in Fig.5(a),all the samples before leaching consist of MgO,TiB2,NaCl and Mg3B2O6 phases. The diffraction peaks of MgO in the four samples are the strongest,and MgO is the main phase of the product. However,the relative intensity of diffraction peak of NaCl is different as the k values vary. The intensity of diffraction peak of NaCl becomes stronger gradually with the increase of k. In addition,all products also contain a small amount of Mg3B2O6 phase with relatively weak diffraction peak. After leaching,the products are mainly composed of TiB2 and Mg3B2O6,as shown in Fig.5(b). The phases of MgO and residual diluent NaCl are not observed because of the removal of them in the process of leaching. Both the relative intensity of the diffraction peak shows that when the value of k is in the range of 0.5 to 1.5,diffraction peak of Mg3B2O6 are all weak. However,when k increases to 2.0,diffraction peak of Mg3B2O6 becomes stronger.
 | Fig.5 XRD patterns of product with different addition of diluent (a)before leaching;(b)after leaching | |
Green samples were preheated inside the reaction chamber of SHS reactor. When the temperature reached about 240℃,the ignition agents began to react and released a lot of heat. The adjacent reactant was heated to high temperature rapidly. When the temperature reached 723K,the reactant B2O3 began to melt. When the temperature continued to rise to 923K,the reducing agent Mg melted. The TiO2 and NaCl in solid state were surrounded quickly by the liquid B2O3 and Mg. At the same time,B2O3 and Mg fully contacted,while redox reaction began to take place and generated intermediate product B. The reaction formula was shown in (4):
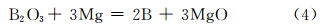
The reaction formula (4) was a strong exothermic reaction which released large amount of heat. The temperature of raw materials rose rapidly. When the temperature reached 1074K,NaCl began to melt,forming a liquid reaction medium. At the same time,due to the strengthening of the quality transmission,Mg liquid and TiO2 were in full contact with each other,leading to the redox reaction of Mg liquid and TiO2 to generate intermediate Ti,as indicated in reaction formula (5).
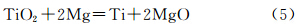
Reaction medium of NaCl liquid promoted the reaction (4) and (5),and the intermediate product B and Ti were rapidly combined to form the final product TiB2. The reaction formula (6) was as follows.

Exothermic reaction (4),(5) and (6) rapidly released a lot of heat,which transferred through heat conduction to adjacent unreacted materials,and the green sample temperature suddenly rose. When the temperature reached the ignition temperature,the reaction (4),(5) and (6) also occurred. The combustion wave moved from the reaction zone to the unreacted zone by self-propagating. Simultaneously,due to the heat release,the temperature of the prior reaction zone decreased to room temperature.
Diluent significantly affected the average particle size of the product based on three points: reducing the combustion temperature of the system,restraining the particles from growing and sintering,and providing liquid medium for the formation of the particles. When reaching oversaturation,the resulting particles would precipitate from the melt and had difficulty in recrystallization,so that the growth would be inhibited. When the temperature was below the melting point of the diluent,the residual volatile diluent was coagulated,forming coating layer on the surface of the particles,which became an obstacle to the quality transmission. Thus,it was difficult for those particles in the melt to grow up.
3 ConclusionsExperimental results show that the introduction of soluble salt into mixture precursor can effectively prevent the aggregation of particles and forming submicron TiB2. The average particle size of the powder product decreases with the increase of the k moles of the diluent. NaCl provides the liquid medium for the combustion synthesis reaction,promotes the mass transfer during the reaction,and improves the conversion rate of TiO2 and product purity. Submicron powders TiB2 can be prepared on a large scale via this self-propagating high-temperature combustion synthesis.
| [1] | SUBRAMANIAN C T S R, MURTHY C H, SURI A K. Synthesis and consolidation of titanium diboride[J]. International Journal of Refractory Metals & Hard Materials, 2007, 25(4): 345-350. |
| [2] | KRISHNARAO R V, SUBRAHMANYAM J. Studies on the formation of TiB2 through carbothermal and B2O3 reduction of TiO2[J]. Materials Science and Engineering: A, 2003, 362:145-151. |
| [3] | KHANRA A K, GODKHINDI M M, PATHAK L C. Sintering behaviour of ultra-fine titanium diboride powder prepared by self-propagating high-temperature synthesis (SHS) technique[J]. Materials Science and Engineering: A, 2007, 454-455: 281-287. |
| [4] | BILGI E, CAMURLU H E, AKGUN B, et al. Formation of TiB2 by volume combustion and mechanochemical process[J]. Materials Research Bulletin, 2008, 43: 873-881. |
| [5] | WELHAM N J. Formation of TiB2 from rutile by room temperature ball milling[J]. Minerals Engineering, 1999, 12: 1213-1224. |
| [6] | MUNRO R G. Material properties of titanium diboride[J]. Journal of Research of the National Institute of Standards and Technology, 2000, 105 (5): 709-720. |
| [7] | SUNDARAM V, LOGAN K V, SPEYER R F. Reaction path in the magnesium thermite reaction to synthesize titanium diboride[J]. Journal of Materials Research, 1997, 12 (10): 2657-2664. |
| [8] | HUANG Y, LEE J K. Preparation of TiB2 powders by mechanical alloying[J]. Materials Letters, 2002, 54(1) : 1-7. |
| [9] | LU L, LAI M O, SU Y, et al. In situ TiB2 reinforced A1 alloy composites[J]. Scripta Materialia, 2001, 45(9) : 1017-1023. |
| [10] | RADEV D D, MARINOV M. Properties of titanium and zirconium diborides obtained by self-propagated high-temperature synthesis[J]. Journal of Alloys and Compounds, 1996, 244(1-2) :48-51. |
| [11] | XIANG X, QING Y. Progress in TiB2 and its composites[J]. Journal of Ceramics, 1999, 20(2): 112-117. |
| [12] | TANG Wen-ming, ZHENG Zhi-xiang, WU Yu-cheng, et al. Synthesis of TiB2 nanocrystalline powder by mechanical alloying[J]. Transactions of Nonferrous Metals Society of China, 2006, 16 : 613—617. |
| [13] | LIU Guang-hua, YANG Kun, LI Jiang-tao, et al. Combustion synthesis of nanosized β-SiC powder on a large scale[J]. Journal of Physical Chemistry C, 2008, 112 (16) : 6285-6292. |
| [14] | NEKAHI ATIYE, FIROOZI SADEGH. Effect of KCl, NaCl and CaCl2 mixture on volume combustion synthesis of TiB2 nanoparticles[J]. Materials Research Bulletin, 2011, 46: 1377-1383. |
| [15] | KHANRA A K, PATHAKB L C, MISHRAB S K,et al. Effect of NaCl on the synthesis of TiB2 powder by a self-propagating high-temperature synthesis technique[J]. Materials Letters, 2004,58: 733-738. |
| [16] | CAMURLU H E, MAGLIA F. Preparation of nano-size ZrB2 powder by self-propagating high temperature synthesis[J]. Journal of the European Ceramic Society, 2009, 29(9):1051-1506. |
| [17] | LA Pei-qing, HAN Shao-bo, JU Qian. Study of the influence of different stoichometry of Mg in staring mixture on particle size and purity of ZrB2 powder prepared by combustion synthesis[J]. Powder Metallurgy Technology, 2013, 31(1) : 1-7. |
| [18] | MERZHANOV A G. Combustion and Plasma Synthesis of High-Temperature Materials[M]. New York: VCH Press, 1990. 204-251. |
 2015, Vol. 43
2015, Vol. 43


