文章信息
- 何志伟, 沈子航, 邱焕逸, 陈家豪, 梁立军, 王建均
- HE Zhi-wei, SHEN Zi-hang, QIU Huan-yi, CHEN Jia-hao, LIANG Li-jun, WANG Jian-jun
- 铝基防冰表面的研究进展
- Research progress in aluminum-based anti-icing surfaces
- 材料工程, 2021, 49(9): 41-50
- Journal of Materials Engineering, 2021, 49(9): 41-50.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2020.000896
-
文章历史
- 收稿日期: 2020-09-28
- 修订日期: 2021-04-13
2. 杭州电子科技大学 光电材料与器件研究中心, 杭州 310018;
3. 杭州电子科技大学 自动化学院, 杭州 310018;
4. 浙江精筑环保科技有限公司, 杭州 310052
2. Center of Advanced Optoelectronic Materials and Devices, Hangzhou Dianzi University, Hangzhou 310018, China;
3. College of Automation, Hangzhou Dianzi University, Hangzhou 310018, China;
4. Zhejiang Jingzhu Environmental Protection Technology Co., Ltd., Hangzhou 310052, China
在长时间低温情况下,积冰在裸露表面的累积往往是不可避免的。例如,输电塔、风力发电机叶片、石油钻井平台、飞机、运输工具等[1-2]表面厚厚的积冰除了可能会增加设备负荷、消耗额外能源外,还可能会导致设备运转不良,甚至产生不可逆的损坏。2008年初,我国南方地区发生了大范围的冰冻雨雪灾害,积雪/冰对交通道路、水管和电网等基础设施造成了极大的破坏,给日常的生产和生活带来诸多不便,造成了巨大的经济损失。为了应对这种低温下的冰冻灾害,实际中较可行的方法为采用防冰措施,主要包括主动法和被动法。其中,主动法包含人工/机械除冰、加盐除冰、加热融冰等,这些方法具有较大的局限性,往往耗能高且可能会造成潜在的环境污染和操作安全等问题[1-2]。目前较有效的防冰方法为被动法,即通过引入防冰表面来实现防冰的目的。这种方法不仅成本低,而且使用寿命较长,对低温下裸露表面的实际防冰应用具有重要意义[2-7]。
防冰表面需要满足以下至少一方面的性能:(1)水滴在结冰前能够自动离开表面;(2)可以延迟水滴在表面的结冰;(3)降低水滴在表面的冰黏附强度[8-10]。到目前为止,防冰策略主要包括超疏水表面[11-13]、水润滑表面[14]、有机润滑表面[15]、低弹性模量表面[9]、裂纹促进表面[8, 10]、聚电解质刷[16]等。由于金属铝资源十分丰富,且有多种优良性能,被广泛应用于不同的设备与运输工具,因此低温地区亟须具有防冰功能的铝基设备表面。本文首先综述了铝基防冰表面的制备方法,包括铝基表面微纳米结构的制备和表面低能化的方法。铝基表面微纳米结构的制备方法包括阳极氧化、酸刻蚀、碱刻蚀、盐刻蚀、沸水处理、喷砂、高速电火花切割等手段;铝基表面的进一步低能化包括微纳米结构表面的化学改性和润滑层的添加,从而分别获得超疏水型和润滑型铝基防冰表面。然后阐述了表征铝基表面防冰性能的两种手段,包括水滴延迟结冰和冰黏附强度,根据水滴延迟结冰时间或冰黏附强度可大致判断不同铝基表面的防冰性能。最后对目前铝基防冰表面的现状进行总结,并对其未来的研究方向和应用进行展望。
1 铝基防冰表面的制备目前铝基防冰表面的制备思路(如图 1所示)可分为两类,包括超疏水型和有机润滑型。这两种类型铝基防冰表面的制备都需要先在表面构建粗糙的铝基微纳米结构,并用低表面能物质来修饰铝基微纳米结构来获得超疏水表面,从而达到排除水滴、延迟水滴结冰或者降低冰黏附强度的目的;或者根据仿猪笼草的润滑机制[15],在铝基表面微纳米粗糙结构上注入带有较强附着力的有机润滑液,达到疏水/冰的目的[17-18]。结合已有文献报道,铝基防冰表面粗糙微纳米结构的主要制备方法有阳极氧化法、酸刻蚀法、盐刻蚀法、热/沸水法、碱刻蚀法、水热法、喷砂法、激光法、溶胶-凝胶法及高速电火花法等,对这些制备方法进行总结,并比较其优缺点,如表 1所示[3, 17-72]。
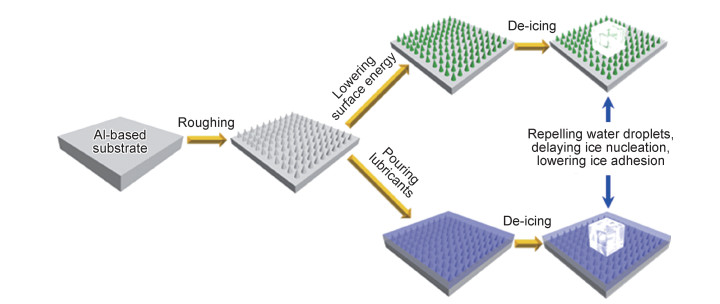
|
图 1 铝基防冰表面的制备及防冰机理 Fig. 1 Preparation and anti-icing mechanism of Al-based anti-icing surfaces |
| Method for preparation of micro/nanostructures | Advantage | Disadvantage | Reference |
| Anodization | Structures controlled | Long reaction time | [17-33] |
| Acid solution | Facile, fast | Structures uncontrolled, destructive | [18, 33-48] |
| Salt solution | Facile, low-cost | Pre-treatment of oxide layers | [3, 46-47, 49-52] |
| Alkaline solution | Facile, fast | Only nanoscale structures | [49, 53-58] |
| Hot/boiling water | Facile, low-cost | Long reaction time | [52-53, 59] |
| Sand-blasting | Efficient | Only microstructures | [57-58, 60-63] |
| Laser | Structures precisely controlled | Costive, small scale | [64-70] |
| Sol-gel | Facile | Potential solvent pollution | [69, 71] |
| High-speed wire cut electrical discharge machining | Structures controlled | Inefficient | [72] |
阳极氧化法是将铝或者铝合金作为阳极,在电解液中通过电化学腐蚀的方法使阳极表面形成粗糙的铝基微纳米结构氧化膜。
Sun等[17]利用阳极氧化法先将经砂纸打磨过的铝片置于0.2 mol/L的氯化钠溶液中刻蚀10 min获得粗糙的铝基微米结构,然后置于0.3 mol/L草酸溶液中刻蚀获得铝基纳米结构,最后用含1%(质量分数,下同)氟硅烷的乙醇溶液在室温下浸泡1 h获得超疏水铝基表面,或者将硅油附着在铝基微纳米结构表面获得润滑型铝基防冰材料。在环境温度为-5 ℃时,该润滑型铝基防冰表面能排除以2000 μL/min下落的冷水滴(2.5 ℃)长达20 min,且其冰黏附强度仅为13.4 kPa,显示了较好的防冰性能[17]。Zhang等[19]以99.5%工业铝箔为阳极,高纯度镍片为阴极,置于2.6 mol/L磷酸和1.6 mol/L甘油混合液(70 ℃)中以3.2 mA/cm2的恒定电流作用2 h,然后用70 ℃的硬脂酸溶液处理1 h,最后对处理过的铝片在80 ℃加热30 min,获得了能有效延迟水滴结冰的超疏水型铝基表面。Boinovich等[20]将铝线在磷酸溶液里阳极氧化,并用功能性氟硅烷进行表面低能化,得到含超疏水表面的铝线(接触角(165±4)°),且该超疏水型铝基表面在(-8.5±0.5) ℃时的轴向剪切冰黏附强度为(113±26) kPa。
由于铝基表面较活泼,阳极氧化法能够通过控制外加电流的大小来控制不同微纳米结构的氧化膜,在该氧化膜上附着有机润滑液或者将其用低能分子处理,即可得到具有防冰性能的铝基表面。
1.2 酸刻蚀法酸刻蚀法是一种较简便的方法,通常只需将铝基表面静置于一定浓度的酸溶液中一定时间,并通过表面低能化即可得到超疏水型铝基防冰表面。
Jin等[45]将铝导线置于20%盐酸溶液中刻蚀1 min,随后将经酸刻蚀的铝导线置于含1%硬脂酸的酒精溶液中浸泡15 min,最后在90 ℃烘箱中烘干,即可得到超疏水铝导线。在-5 ℃和相对湿度85%的情况下,经过110 min的防冰测试,该超疏水铝导线上也只有一小部分冰,体现了较好的防冰效果[45]。Ganne等[33]将铝合金网或片置于氢氟酸和盐酸混合液中1~5 min,经氟硅烷低能化处理后得到超疏水铝基防冰表面,该表面在-10 ℃时能有效延迟水滴结冰。Barthwal等[18]在室温下将铝合金片置于盐酸溶液(33%,体积分数)中刻蚀3 min,获得铝基微米结构,然后在1 mol/L的硫酸溶液里进行阳极氧化得到铝基纳米结构,再用聚二甲基硅氧烷进行表面修饰并在硅油中浸泡20 min,最终获得润滑型防冰表面。-10 ℃时,该表面的冰黏附强度低至(22±5) kPa[18]。
该方法也易结合其他微纳米结构制备方法,但所制得的微纳米结构可控性不足,且酸腐蚀对铝基表面的破坏性较大。
1.3 盐刻蚀法盐刻蚀法主要利用金属铝的活泼性,通过和盐溶液进行置换反应,制备铝基微纳米结构来获得超疏水型防冰表面。
占彦龙等[3]先用3000目砂纸打磨获得微米级的粗糙结构,经无水乙醇和超声清洗后置于0.05 mol/L的硫酸铜溶液中,然后在5%的月桂酸酒精溶液中浸泡1.5 h,获得接触角为163.31°且滚动角小于5°的超疏水铝基表面。其中用硫酸铜盐溶液刻蚀80 min得到的超疏水型铝基防冰表面表现出了最佳的延迟水滴结冰性能,其延迟时间达到普通铝表面的5倍[3]。Chu等[50]将铝箔浸泡在0.1 mol/L盐酸和0.1 mol/L硝酸铜混合溶液中5 min,并用1%氟硅烷低能化30 min,获得超疏水型铝基防冰表面,并研究了水滴在该超疏水表面的二次结冰作用机制。Liao等[47]将铝箔分别置于1 mol/L CuCl2溶液中8 s和盐酸溶液中10 s,刻蚀后的铝箔用2%三甲基硅氧烷进行表面低能化,获得接触角为161.9°且滚动接触角为6.8°的超疏水型铝基防冰表面,该表面能延迟水滴结冰长达475 s。
该方法使用前一般须进行预处理,即除去铝基表面的氧化膜,且所使用盐溶液里的金属离子能够与铝基表面发生反应,从而生成相应的金属基微纳米结构。
1.4 碱刻蚀法碱刻蚀法是基于铝基表面与碱溶液发生的反应,该反应能够在表面生成纳米级的铝基薄片,经过必要的表面低能化后即可获得超疏水型铝基防冰表面。
Xu等[55]将1060铝合金网置于0.005 mol/L的NaOH溶液中120 min,使其表面粗糙化,随后在室温下将刻蚀后的铝合金网用氟硅烷进行表面低能化,获得超双疏型铝基防冰表面,将0.05 mL水滴放置在该表面并置于-18 ℃冰箱中,其能够延迟水滴结冰长达26 h,表现出了优异的防冰性能。Wang等[57]先对铝片(99.9%)进行喷砂处理,然后在0.1 mol/L的NaOH溶液(80 ℃)中浸泡5 min及在沸水中浸泡40 min,最后用1% 氟硅烷的乙醇溶液进行低能化24 h,获得超疏水型防冰表面,在-15 ℃和湿度78%的情况下,该防冰表面能够有效延迟水滴结冰长达81 min。Shen等[58]先对铝合金片喷砂得到粗糙微米结构,然后在0.05 mol/L的NaOH溶液(80 ℃)中浸泡5 min,最后用氟硅烷溶液进行低能化从而获得超疏水型防冰表面,在-10 ℃时,该防冰表面的冰黏附强度为75 kPa,且能够延迟水滴结冰达到769 s。
该方法较为简便,可以结合其他制备方法,在已有铝基微米结构的基础上制备铝基纳米结构,例如酸刻蚀法、喷砂法等制备方法。
1.5 热/沸水法热/沸水法能使铝基表面的铝酸盐离子结晶形成一层较稳定的氢氧化铝,众多的羟基易于与低能表面物质反应,形成一层低表面能的分子[53]。
Zuo等[52]将铝箔分别置于1 mol/L CuCl2溶液中8 s及90 ℃的热水中50 min,随后用2%三甲基硅氧烷溶液对铝基表面进行低能化,并获得接触角为(164.8±1.1)°和滚动接触角小于1°的铝基超疏水表面,该铝基防冰表面在-6 ℃时能延迟水滴结冰超过110 min。Kim等[53]先将铝片(99.5%)置于0.05 mol/L NaOH溶液(80 ℃)中5 min及沸水中30 min,使其表面形成铝基纳米结构,并用氟硅烷进行表面低能化获得超疏水型防冰表面,在水蒸气过饱和度为3.41时,该表面能有效防霜冻长达1779 s。Han等[59]将0.1 mm厚的纯铝片置于100 ℃的沸水中超过40 min,并用十八烷基三氯硅烷进行表面低能化获得超疏水型防冰表面,在-20 ℃时该表面能够延迟水滴结冰超过2 h。
该方法操作简单,可单独使用制备铝基纳米结构,也可以结合碱刻蚀法、酸刻蚀法等共同使用来控制铝基微纳米结构。
1.6 喷砂法使用喷砂法的主要目的是使铝基表面获得微米级的粗糙结构,铝基微米结构可通过喷砂颗粒粒径和压力进行调节,并通过进一步的纳米结构修饰和表面低能化来获得铝基防冰表面。
Zou等[60]将喷嘴置于AA2024 Al表面10 cm处,在240 kPa压力下将平均粒径165 μm的氧化铝离子喷射上去(时间为10 s),再通过沉积硅氧烷膜获得疏水型铝基防冰表面,该表面在-10 ℃时的冰黏附强度最低达160 kPa。Wang等[57]在干净铝片(99.9%)上,以0.5 MPa的压力喷射150目氧化铝,持续时间为30 min,得到约50 μm可调粗糙度的表面,然后用0.1 mol/L氢氧化钠溶液刻蚀5 min及用沸水浸泡40 min,最后用1%氟硅烷乙醇溶液进行表面低能化,获得超疏水型防冰表面,该表面能够延迟水滴结冰长达81 min。Balordi等[62-63]将洗净的铝合金置于喷嘴5 cm处,使用3.5×105 Pa的压力持续喷砂10 s,将微米级的玻璃珠喷射在铝合金表面,获得一层微米级粗糙的涂层,然后在100 ℃超纯水中煮沸5 min,并用氟硅烷处理获得超疏水型和润滑型铝基防冰表面,其剪切冰黏附强度分别在100 kPa和50 kPa左右,表现出了较好的防冰性能[63]。
该方法单独使用只能制备微米级粗糙表面,这比较利于制备润滑型防冰表面。如需制备超疏水型防冰表面,还需结合其他常用方法做进一步纳米修饰。
1.7 激光法激光法利用高能激光光束照射来刻蚀铝基表面,使其形成一定深度的凹槽,该方法具有刻蚀成功率高、稳定性高、无污染等优点,适合于有高精度需求的超疏水型防冰表面的制备。
Liu等[64]在洗净的7075铝合金片上用激光刻蚀两次,刻蚀区域为10 mm ×10 mm,平均功率为50 W,重复频率为20 kHz,脉冲宽度为200 ns,扫描速率为500 mm/s,并用0.01 mol/L硬脂酸溶液处理,获得超疏水型防冰表面,该表面在-15 ℃时能够延迟水滴结冰长达1938 s,表现出了较好的防冰性能。Xing等[65]在经抛光的5052铝合金表面(20 mm×20 mm×5 mm)采用波长为1030 nm的线性极化皮秒激光进行刻蚀,其重复频率为500 kHz,脉冲宽度为500 ps,输出功率为20 W,扫描速率为3000 mm/s,扫描的线长度在20~100 μm之间,该表面从0 ℃降至-23 ℃时,能够延迟水滴结冰长达4253 s,表现出了较好的防冰性能。Li等[66]在经抛光后的7075铝合金表面采用激光刻蚀,平均功率为50 W,重复频率为20 kHz,脉冲宽度为200 ns,扫描速率为500 mm/s,然后用1%的氟硅烷进行低能化和PDMS填充凹槽,获得超疏水型防冰表面,该表面的冰黏附强度最低可至(69±20) kPa。
该方法较简便,且能够较精确地控制微纳米尺寸、形状、间距和纵深,可对除冰后微纳结构表面破坏情况进行综合分析,有利于研究超疏水型防冰表面的除冰机理,从而改进防冰表面微纳米结构的设计。
1.8 其他其他制备方法,如溶胶-凝胶法、高速电火花法、水热法等往往和上述常用方法结合使用来制备铝基微纳米结构,通过一定的表面低能化来获得超疏水型防冰表面。相对于常用的阳极氧化、酸刻蚀和碱刻蚀等方法来讲,这些方法所制备的铝基微纳米结构表面在防冰领域应用较少,仍有待于后期继续深入地研究。
2 铝基防冰表面防冰能力的表征随着时间累积和温度降低,在铝基表面水结冰的现象往往是不可避免的。因此,防冰表面一般通过延迟水滴结冰或者降低冰黏附强度来实现防冰的目的。
2.1 延迟结冰水滴在微纳米结构上的润湿状态通常包括Wenzel态(见图 2(a)),Cassie-Baxter态(见图 2(b))及两态之间的混合态。从热力学的角度看,在降温过程中,可以把微纳米结构上的水滴看成一个固-液-气三相的体系。水滴以接触热传导和热辐射的方式从空气中获得热量;也可通过与冷表面接触热传导和热辐射的方式失去热量[73-75]。对于微纳米结构表面来讲,如果水滴在其表面呈Cassie-Baxter态,则空气存在于水滴与微纳米结构之间(见图 2(b)),其能够有效地阻止热辐射,从而延长水滴结冰时间;当水滴在微纳米结构表面呈现Wenzel态时,水滴直接与冷表面及微纳米结构接触,易通过接触式传导的方式损失热量。因此,相较于Cassie-Baxter态,Wenzel态水滴延迟结冰的时间变短。对于有机润滑铝基防冰表面,水滴与有机润滑液层之间不存在空气,其延迟结冰的能力主要基于有机润滑层本身的低表面能性质。除了热传导之外,在水滴被冷冻的过程中,微纳米结构之间可能会存在极小的水滴,这部分极小的水滴容易在微纳米结构的边界或者缺陷处冷冻为冰,也可能通过弹跳的方式离开表面[76]。另外,如果水滴在铝基防冰表面进行反复的冷冻和解冻,防冰表面的微纳米结构或者有机润滑层会随之损耗[77-78],其延迟水滴结冰的能力也将随之降低。
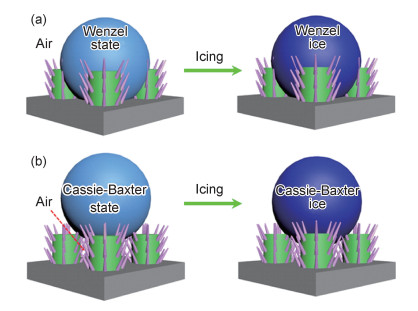
|
图 2 超疏水表面水滴的润湿行为(a)和冷冻行为(b) Fig. 2 Wetting (a) and freezing (b) behaviors of water droplets on superhydrophobic surfaces |
对于水滴延迟结冰的表征,目前尚未统一的衡量标准,需依据铝基防冰表面的种类、水滴大小、相对湿度、测试温度等因素综合来判断铝基表面延迟水滴结冰的能力。表 2为超疏水型铝基防冰表面的水滴延迟结冰总结情况[3, 8, 24, 39-41, 44, 47, 56-58, 61, 64-65, 71],可以看出,测试水滴的大小在4~50 μL之间,相对湿度为47%~90%,测试温度为16.5~-23 ℃,延迟时间在300~7500 s之间。在延迟水滴结冰过程中,表 2中的测试温度也存在两种情况:(1)将水滴直接置于目标温度环境中;(2)将水滴从室温逐渐冷却,直至达到目标温度。由于延迟结冰的条件不同,简单地通过比较延迟时间来比较不同表面防冰性能优劣的意义不大,目前研究人员也没有提出明确的延迟时间作为判断防冰表面的依据,因此结冰延迟时间仅可作为判断同类表面防冰性能的参考指标[74]。
| Type of Al-based surfaces | Drop size/μL | Relative humidity/% | Temperature/℃ | Delayed time/s | Reference |
| Al/oleic acid | 6 | -12±1 | 1604 | [24] | |
| Al alloy/FAS-17 | 45 | -6--10 | 900 | [39] | |
| 6205 Al alloy/FAS-17 | 10 | -10±0.1 | 2280 | [40] | |
| Al/stearic acid | 8 | 60±5 | -10 | 380 | [56] |
| 1060 Al/HDTMS | 10 | 77±10 | -7 | 475 | [47] |
| Al/organic acid | 4 | 47-83 | 16.5--10 | 3940 | [71] |
| 6061 Al/ATPS | 5 | > 80 | 0--12.2 | 1417 | [61] |
| 6063 Al alloy/stearic acid | 20 | -8 | 1500 | [44] | |
| Al/CuO/lauric acid | 10 | 68 | 12.8--10 | 300 | [3] |
| Al/FAS-17 | 5 | 90 | -10 | 7500 | [8] |
| 5052 Al alloy | 50 | 0--23 | 4253 | [65] | |
| Al/FAS-17 | 4 | 78 | -15 | 4860 | [57] |
| Al alloy/stearic acid | 20 | -15 | 2365 | [41] | |
| Al alloy/FAS-17 | 4 | 65 | -10 | 769 | [58] |
| 7075 Al alloy/stearic acid | 5 | 53±5 | 16--15 | 1938 | [64] |
冰黏附强度可用单位接触面积上除冰作用力的最大值来定义,其影响因素有很多,例如冰黏附强度测试方法[79]、测试温度[79]、测试仪器[2]、冰的种类[80]、冰的大小[81]、表面类型[82]、冰的断裂方式[81]等。通常情况下,对于冰黏附强度而言,不考虑测试冰块大小对它的影响,且冰在表面的断裂方式为机械黏合失效。此外,除冰测试设备往往依赖于测试方法,而冰的类型取决于结冰温度和测试方法,故测试方法和测试温度确定,所测得的冰黏附强度就具有可比性[81]。
表 3总结了不同铝基防冰表面冰黏附强度的测试方法和测试温度[4, 17-18, 20-22, 25, 28, 30, 34, 36-38, 58, 60, 62-63, 66, 83-84]。可以看出,冰黏附强度在0.58~300 kPa之间,其测试温度在-5~-33 ℃之间,测试方法则有离心力法、轴向剪切力法、水平剪切力法、垂直剪切力法和抗张强度法(见图 3)。铝基有机润滑防冰表面(见图 1)具有较低的冰黏附强度(< 30 kPa)[17-18],然而反复结冰/除冰过程中有机润滑层的损耗不可避免,故该类表面的使用期限取决于有机润滑层的存在与否[77]。对于超疏水型铝基防冰表面(见图 1)而言,其冰黏附强度主要取决于低能表面层的可持续性和微纳米结构的强度。在长时间低温和较高湿度的情况下,冰在微纳米结构间隙中会不断形成,并与微纳米结构形成互锁的状态[85-86],这会显著增加表面的冰黏附强度。在反复除冰过程中,超疏水型铝基防冰表面的低能表面层和微纳米结构会渐渐地损耗,最终失去防冰性能。
| Type of Al-based surfaces | Test method | Temperature/℃ | Ice adhesion strength/kPa | Reference |
| 6061 Al alloy/PTFE | Centrifugal | -10 | 72±12 | [21] |
| 6061 Al alloy/HMDSO | Centrifugal | -10±1 | 100±9 | [22] |
| 6061 Al alloy/FS-300 | Centrifugal | -10 | 103 | [25] |
| Al/FAS-17 | Axial shear | -8.5±0.5 | 113±26 | [20] |
| Al/myristic acid | Horizontal shear | -10 | 65±22 | [28] |
| AA1060H24 Al/PTES | Tensile | -10 | 200 | [34] |
| 6061 Al alloy/PTFE | Centrifugal | -10 | 188±12 | [36] |
| Al/FAS | Tensile | -6 | 0.58 | [37] |
| Al/PFOTES | Tensile | -9 | 20 | [38] |
| AA2024 Al alloy/FAS | Centrifugal | -10 | 86.2±29 | [4] |
| AA2024 Al alloy/HMDSO/C4F8 | Horizontal shear | -10 | 160 | [60] |
| Al/silicone oil | Vertical shear | -5 | 14.4 | [17] |
| Al alloy/FAS-17 | Horizontal shear | -10 | 75 | [58] |
| 6082 Al alloy/FAS | Axial shear | -19 | 62 | [62] |
| 6082 Al alloy/Dynasilan®9896 | Axial shear | -19 | 45 | [63] |
| 6061 Al alloy/silicone oil | Horizontal shear | -10 | 22±5 | [18] |
| Al/FAS-17 | Horizontal shear | -10 | 35.7 | [30] |
| 7075 Al alloy/FAS-13/PDMS | Horizontal shear | -33±2 | 69±20 | [66] |
| AA6061 Al/FDDTS | Horizontal shear | -10 | 300 | [83] |
| Al2024 T4/POTS | Centrifugal | -10 | 117 | [84] |
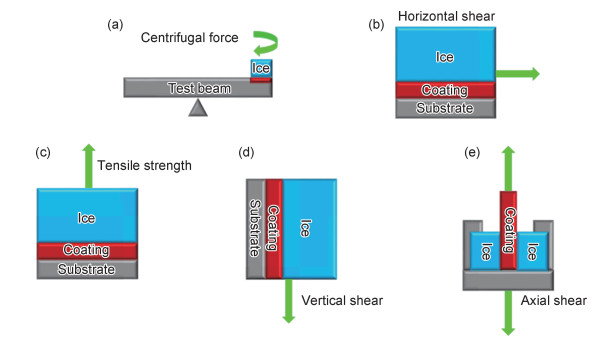
|
图 3 常用检测冰黏附强度的方法 (a)离心力;(b)水平剪切力;(c)抗张强度;(d)垂直剪切力;(e)轴向剪切力 Fig. 3 Most commonly used test methods for ice adhesion strength (a)centrifugal force; (b)horizontal shear; (c)tensile strength; (d)vertical shear; (e)axial shear |
在长时间低温的情况下,材料表面结冰的累积往往是不可避免的,因此如何降低防冰表面的冰黏附强度或者延迟表面水滴结冰成为目前的研究热点。虽然主动除冰法已被广泛应用于社会生产和生活中,但其花费巨大,可能会产生环境污染,也存在潜在的安全隐患。被动除冰法,则能有效地降低结冰的可能性,或者使表面积冰被轻易除去。铝基材料广泛应用于生产和生活中,研究低温下铝基表面的防冰性能有助于各种设备、设施安全可靠地运行。本文主要介绍了两种类型的仿生铝基防冰表面:有机润滑型表面和超疏水型表面。对于这两种表面的制备,都需先在铝基表面制备微纳米结构,再通过表面低能化或者填充有机润滑液来实现。然后介绍了各种微纳米结构的制备方法及其优缺点,包括阳极氧化法、酸刻蚀法、盐刻蚀法、碱刻蚀法、热/沸水法、喷砂法、激光法、溶胶-凝胶法、高速电火花法等。最后阐述了铝基防冰表面的两种表征方式:冰黏附强度和水滴延迟结冰。通过表征手段,在一定程度上能够大致判断某种铝基表面是否具有防冰性能。
目前,铝基防冰表面和其他类型的防冰表面一样,如何增加铝基表面的耐磨性及结冰/除冰循环下表面防冰的可持续性是实际应用中亟须解决的难题。对于有机润滑型铝基防冰表面来讲,有机润滑层本身的机械耐磨性不佳,其在结冰/除冰反复循环过程中会逐渐地被积冰带走,从而产生不可逆的损耗。目前较为可行的方式为改变润滑层液体种类或者使用固体有机润滑层(低温下为固体),从而来延长其实际应用中的使用寿命。对于超疏水型铝基防冰表面,可通过增加微纳米结构本身的强度、设计更加合理且具有力学强度的微纳米结构、提高微纳米结构本身的弹性、利用微纳米结构的自愈合性能以及材料内部引入不同尺度的中空结构等方式来提高其表面的耐磨性及低能表面层的可持续性,从而延长其实际应用中防冰性能的持续时间。总体来说,铝基防冰表面能够减缓铝基设备表面的积冰形成或者降低其冰黏附强度,保障了设备在低温下长时间运行的安全性和可靠性,也为未来铝基防冰表面在南北极勘探、海洋开发、电动车低温节能等方面发挥重要作用提供了可能。
| [1] |
HE Z, ZHUO Y, WANG F, et al. Design and preparation of icephobic PDMS-based coatings by introducing an aqueous lubricating layer and macro-crack initiators at the ice-substrate interface[J]. Progress in Organic Coatings, 2020, 47: 105737. |
| [2] |
HE Z, VÅGENES E T, DELABAHAN C, et al. Room temperature characteristics of polymer-based low ice adhesion surfaces[J]. Scientific Reports, 2017, 7: 42181. DOI:10.1038/srep42181 |
| [3] |
占彦龙, 李文, 李宏, 等. 氧化还原法制备超疏水表面及其防覆冰性能[J]. 材料工程, 2019, 47(1): 58-63. ZHAN Y L, LI W, LI H, et al. Fabrication of superhydrophobic surface by redox process and its anti-icing performance[J]. Journal of Materials Engineering, 2019, 47(1): 58-63. |
| [4] |
KULINICH S A, HONDA M, ZHU A L, et al. The icephobic performance of alkyl-grafted aluminum surfaces[J]. Soft Matter, 2015, 11(5): 856-861. DOI:10.1039/C4SM02204A |
| [5] |
YU Y, JIN B, JAMIL M I, et al. Highly stable amphiphilic organogel with exceptional anti-icing performance[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12838-12845. |
| [6] |
LV J, ZHU C, QIU H, et al. Robust icephobic epoxy coating using maleic anhydride as a crosslinking agent[J]. Progress in Organic Coatings, 2020, 142: 105561. DOI:10.1016/j.porgcoat.2020.105561 |
| [7] |
RICO V, MORA J, GARCÍA P, et al. Robust anti-icing superhydrophobic aluminum alloy surfaces by grafting fluorocarbon molecular chains[J]. Applied Materials Today, 2020, 21: 100815. DOI:10.1016/j.apmt.2020.100815 |
| [8] |
HE Z, XIAO S, GAO H, et al. Multiscale crack initiator promoted super-low ice adhesion surfaces[J]. Soft Matter, 2017, 13(37): 6562-6568. DOI:10.1039/C7SM01511A |
| [9] |
HE Z, ZHUO Y, HE J, et al. Design and preparation of sandwich-like PDMS sponges with super-low ice adhesion[J]. Soft Matter, 2018, 14(23): 4846-4851. DOI:10.1039/C8SM00820E |
| [10] |
HE Z, ZHUO Y, WANG F, et al. Understanding the role of hollow sub-surface structures in reducing ice adhesion strength[J]. Soft Matter, 2019, 15(13): 2905-2910. DOI:10.1039/C9SM00024K |
| [11] |
YU C, GUICHENG L, LEI J, et al. Icephobic performance on the aluminum foil-based micro-/nanostructured surface[J]. Chinese Physics B, 2017, 26(4): 046801. DOI:10.1088/1674-1056/26/4/046801 |
| [12] |
HE Z, HE J, ZHANG Z. Selective growth of metallic nanostructures on microstructured copper substrate in solution[J]. Cryst EngComm, 2015, 17(38): 7262-7269. |
| [13] |
HE Z, ZHANG Z, HE J. CuO/Cu based superhydrophobic and self-cleaning surfaces[J]. Scripta Materialia, 2016, 118: 60-64. DOI:10.1016/j.scriptamat.2016.03.015 |
| [14] |
CHEN J, LUO Z, FAN Q, et al. Anti-ice coating inspired by ice skating[J]. Small, 2014, 10(22): 4693-4699. DOI:10.1002/smll.201401557 |
| [15] |
WONG T S, KANG S H, TANG S K Y, et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity[J]. Nature, 2011, 477(7365): 443-447. DOI:10.1038/nature10447 |
| [16] |
CHERNYY S, JÄRN M, SHIMIZU K, et al. Superhydrophilic polyelectrolyte brush layers with imparted anti-icing properties: effect of counter ions[J]. ACS Applied Materials & Interfaces, 2014, 6(9): 6487-6496. |
| [17] |
SUN J, WANG C, SONG J, et al. Multi-functional application of oil-infused slippery Al surface: from anti-icing to corrosion resistance[J]. Journal of Materials Science, 2018, 53(23): 16099-16109. DOI:10.1007/s10853-018-2760-z |
| [18] |
BARTHWAL S, LEE B, LIM S H. Fabrication of robust and durable slippery anti-icing coating on textured superhydrophobic aluminum surfaces with infused silicone oil[J]. Applied Surface Science, 2019, 496: 143677. DOI:10.1016/j.apsusc.2019.143677 |
| [19] |
ZHANG Y, YU X, WU H, et al. Facile fabrication of superhydrophobic nanostructures on aluminum foils with controlled-condensation and delayed-icing effects[J]. Applied Surface Science, 2012, 258(20): 8253-8257. DOI:10.1016/j.apsusc.2012.05.032 |
| [20] |
BOINOVICH L B, ZHEVNENKO S N, EMEL'YANENKO A M, et al. Adhesive strength of the contact of ice with a superhydrophobic coating[J]. Doklady Chemistry, 2013, 448(2): 71-75. DOI:10.1134/S0012500813020079 |
| [21] |
JAFARI R, MENINI R, FARZANEH M. Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings[J]. Applied Surface Science, 2010, 257(5): 1540-1543. DOI:10.1016/j.apsusc.2010.08.092 |
| [22] |
MOBARAKEH L F, JAFARI R, FARZANEH M. The ice repellency of plasma polymerized hexamethyldisiloxane coating[J]. Applied Surface Science, 2013, 284: 459-463. DOI:10.1016/j.apsusc.2013.07.119 |
| [23] |
JING T, KIM Y, LEE S, et al. Frosting and defrosting on rigid superhydrophobic surface[J]. Applied Surface Science, 2013, 276: 37-42. DOI:10.1016/j.apsusc.2013.02.105 |
| [24] |
SONG M, LIU Y, CUI S, et al. Fabrication and icing property of superhydrophilic and superhydrophobic aluminum surfaces derived from anodizing aluminum foil in a sodium chloride aqueous solution[J]. Applied Surface Science, 2013, 283: 19-24. DOI:10.1016/j.apsusc.2013.05.088 |
| [25] |
MENINI R, GHALMI Z, FARZANEH M. Highly resistant icephobic coatings on aluminum alloys[J]. Cold Regions Science and Technology, 2011, 65(1): 65-69. DOI:10.1016/j.coldregions.2010.03.004 |
| [26] |
BOINOVICH L, EMELYANENKO A M, KOROLEV V V, et al. Effect of wettability on sessile drop freezing: when superhydrophobicity stimulates an extreme freezing delay[J]. Langmuir, 2014, 30(6): 1659-1668. DOI:10.1021/la403796g |
| [27] |
RUAN M, LI W, WANG B, et al. Preparation and anti-icing behavior of superhydrophobic surfaces on aluminum alloy substrates[J]. Langmuir, 2013, 29(27): 8482-8491. DOI:10.1021/la400979d |
| [28] |
ZHENG S, LI C, FU Q, et al. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications[J]. Materials & Design, 2016, 93: 261-270. |
| [29] |
BOINOVICH L B, DOMANTOVSKII A G, EMELYANENKO A M, et al. Anti-icing performance of superhydrophobic coatings on aluminum and stainless steel[J]. Russian Chemical Bulletin, 2013, 62(2): 380-387. DOI:10.1007/s11172-013-0049-6 |
| [30] |
JIN M, SHEN Y, LUO X, et al. A combination structure of microblock and nanohair fabricated by chemical etching for excellent water repellency and icephobicity[J]. Applied Surface Science, 2018, 455: 883-890. DOI:10.1016/j.apsusc.2018.06.043 |
| [31] |
GRIZEN M, MAITRA T, BRADLEY J P, et al. Nanotextured aluminum-based surfaces with icephobic properties[J]. Heat Transfer Engineering, 2020, 41(19/20): 1663-1672. |
| [32] |
郑顺丽, 李澄, 项腾飞, 等. 阳极氧化法制备铝基超疏水涂层及其稳定性和耐蚀性的研究[J]. 材料工程, 2017, 45(10): 71-78. ZHENG S L, LI C, XIANG T F, et al. Fabrication of aluminum-based superhydrophobic coating by anodization and research on stability and corrosion resistance[J]. Journal of Materials Engineering, 2017, 45(10): 71-78. DOI:10.11868/j.issn.1001-4381.2016.000301 |
| [33] |
GANNE A, LEBED V O, GAVRILOV A I. Combined wet chemical etching and anodic oxidation for obtaining the superhydrophobic meshes with anti-icing performance[J]. Colloids and Surfaces A, 2016, 499: 150-155. DOI:10.1016/j.colsurfa.2016.04.019 |
| [34] |
WANG Y, XUE J, WANG Q, et al. Verification of icephobic/anti-icing properties of a superhydrophobic surface[J]. ACS Applied Materials & Interfaces, 2013, 5(8): 3370-3381. |
| [35] |
WANG F, LI C, LV Y, et al. Ice accretion on superhydrophobic aluminum surfaces under low-temperature conditions[J]. Cold Regions Science Technology, 2010, 62(1): 29-33. DOI:10.1016/j.coldregions.2010.02.005 |
| [36] |
SALEEMA N, FARZANEH M, PAYNTER R W, et al. Prevention of ice accretion on aluminum surfaces by enhancing their hydrophobic properties[J]. Journal of Adhesion Science and Technology, 2011, 25(1/3): 27-40. |
| [37] |
WANG F, LV F, LIU Y, et al. Ice adhesion on different microstructure superhydrophobic aluminum surfaces[J]. Journal of Adhesion Science and Technology, 2012, 27(1): 58-67. |
| [38] |
OU J, SHI Q, WANG Z, et al. Sessile droplet freezing and ice adhesion on aluminum with different surface wettability and surface temperature[J]. Science China Physics, Mechanics & Astronomy, 2015, 58(7): 1-8. |
| [39] |
YANG J, LI W. Preparation of superhydrophobic surfaces on Al substrates and the anti-icing behavior[J]. Journal of Alloys and Compounds, 2013, 576: 215-219. DOI:10.1016/j.jallcom.2013.04.060 |
| [40] |
YANG W X, YUAN Y, LIU G Y, et al. The anti-icing/frosting aluminum surface with hydrangea-like micro/nano structure prepared by chemical etching[J]. Materials Letters, 2018, 226: 4-7. DOI:10.1016/j.matlet.2018.04.100 |
| [41] |
TONG W, XIONG D, WANG N, et al. Green and timesaving fabrication of a superhydrophobic surface and its application to anti-icing, self-cleaning and oil-water separation[J]. Surface & Coatings Technology, 2018, 352: 609-618. |
| [42] |
KIM M H, KIM D R, LEE K S. Stochastic approach to the anti-freezing behaviors of superhydrophobic surfaces[J]. International Journal of Heat and Mass Transfer, 2017, 106: 841-846. DOI:10.1016/j.ijheatmasstransfer.2016.10.015 |
| [43] |
BRUCE RALPHIN ROSE J. Advances in mechanical engineering: a feasibility study of superhydrophobic coating on Al6061 for airplane anti-icing applications[M]. Singapore: Springer, 2020: 1663-1674.
|
| [44] |
晏忠钠, 车彦慧, 冯利邦, 等. 超疏水铝合金表面的防覆冰和防黏附行为[J]. 材料工程, 2015, 43(9): 25-29. YAN Z N, CHE Y H, FENG L B, et al. Anti-icing and anti-adhesion behavior of superhydrophobic aluminum alloy surface[J]. Journal of Materials Engineering, 2015, 43(9): 25-29. |
| [45] |
JIN H Y, NIE S C, LI Z W, et al. Investigation on preparation and anti-icing performance of super-hydrophobic surface on aluminum conductor[J]. Chinese Journal of Chemical Physics, 2018, 31(2): 216-222. DOI:10.1063/1674-0068/31/cjcp1707152 |
| [46] |
万闪, 姜丹, 蔡光义, 等. 铝合金超疏水转化膜的制备与性能[J]. 材料工程, 2018, 46(9): 144-151. WAN S, JIANG D, CAI G Y, et al. Preparation and properties of superhydrophobic conversion film on aluminium alloy[J]. Journal of Materials Engineering, 2018, 46(9): 144-151. |
| [47] |
LIAO R, ZUO Z, GUO C, et al. Fabrication of superhydrophobic surface on aluminum by continuous chemical etching and its anti-icing property[J]. Applied Surface Science, 2014, 317: 701-709. DOI:10.1016/j.apsusc.2014.08.187 |
| [48] |
ZHANG M L, CHEN R R, LIU Q, et al. Long-term stability of a liquid-infused coating with anti-corrosion and anti-icing potentials on Al alloy[J]. ChemElectroChem, 2019, 6(15): 3911-3919. DOI:10.1002/celc.201900302 |
| [49] |
SHEN Y, WANG G, ZHU C, et al. Petal shaped nanostructures planted on array micro-patterns for superhydrophobicity and anti-icing applications[J]. Surface & Coatings Technology, 2017, 319: 286-293. |
| [50] |
CHU F, GAO S, ZHANG X, et al. Droplet re-icing characteristics on a superhydrophobic surface[J]. Applied Physics Letters, 2019, 115(7): 073703. DOI:10.1063/1.5109283 |
| [51] |
RODI P, KAPUN B, PANJAN M, et al. Easy and fast fabrication of self-cleaning and anti-icing perfluoroalkyl silane film on aluminium[J]. Coatings, 2020, 10(3): 234. DOI:10.3390/coatings10030234 |
| [52] |
ZUO Z, LIAO R, GUO C, et al. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface[J]. Applied Surface Science, 2015, 331: 132-139. DOI:10.1016/j.apsusc.2015.01.066 |
| [53] |
KIM A, LEE C, KIM H, et al. Simple approach to superhydrophobic nanostructured Al for practical antifrosting application based on enhanced self-propelled jumping droplets[J]. ACS Applied Materials & Interfaces, 2015, 7(13): 7206-7213. |
| [54] |
GWAK Y, PARK J I, KIM M, et al. Creating anti-icing surfaces via the direct immobilization of antifreeze proteins on aluminum[J]. Scientific Reports, 2015, 5: 12019. DOI:10.1038/srep12019 |
| [55] |
XU Z, QI H, CHENG Y, et al. Nanocoating: anti-icing superamphiphobic surface on 1060 aluminum alloy mesh[J]. Applied Surface Science, 2019, 498: 143827. DOI:10.1016/j.apsusc.2019.143827 |
| [56] |
LI W, ZHANG X, YANG J, et al. In situ growth of superhydrophobic and icephobic films with micro/nanoscale hierarchical structures on the aluminum substrate[J]. Journal of Colloid and Interface Science, 2013, 410: 165-171. DOI:10.1016/j.jcis.2013.07.063 |
| [57] |
WANG G Y, SHEN Y Z, TAO J, et al. Facilely constructing micro-nanostructure superhydrophobic aluminum surface with robust ice-phobicity and corrosion resistance[J]. Surface & Coatings Technology, 2017, 329: 224-231. |
| [58] |
SHEN Y Z, WANG G Y, TAO J, et al. Anti-icing performance of superhydrophobic texture surfaces depending on reference environments[J]. Advanced Materials Interfaces, 2017, 4(22): 1700836. DOI:10.1002/admi.201700836 |
| [59] |
HAN S, JEONG J, LEE D. Ice-phobic behavior of superhydrophobic Al surface under various etching conditions[J]. Journal of Electroceramics, 2014, 33(1/2): 82-88. DOI:10.1007/s10832-014-9924-2 |
| [60] |
ZOU M, BECKFORD S, WEI R, et al. Effects of surface roughness and energy on ice adhesion strength[J]. Applied Surface Science, 2011, 257(8): 3786-3792. DOI:10.1016/j.apsusc.2010.11.149 |
| [61] |
YIN L, XIA Q, XUE J, et al. In situ investigation of ice formation on surfaces with representative wettability[J]. Applied Surface Science, 2010, 256(22): 6764-6769. DOI:10.1016/j.apsusc.2010.04.086 |
| [62] |
BALORDI M, CAMMI A, De SANTUCCI MAGISTRIS G, et al. Role of micrometric roughness on anti-ice properties and durability of hierarchical super-hydrophobic aluminum surfaces[J]. Surface & Coatings Technology, 2019, 374: 549-556. |
| [63] |
BALORDI M, De SANTUCCI MAGISTRIS G, CHEMELLI C. A novel simple anti-ice aluminum coating: synthesis and in-lab comparison with a superhydrophobic hierarchical surface[J]. Coatings, 2020, 10(2): 111. DOI:10.3390/coatings10020111 |
| [64] |
LIU Y, LI X, JIN J, et al. Anti-icing property of bio-inspired micro-structure superhydrophobic surfaces and heat transfer model[J]. Applied Surface Science, 2017, 400: 498-505. DOI:10.1016/j.apsusc.2016.12.219 |
| [65] |
XING W, LI Z, YANG H, et al. Anti-icing aluminum alloy surface with multi-level micro-nano textures constructed by picosecond laser[J]. Materials & Design, 2019, 183: 108156. |
| [66] |
LI X, WANG G, ZHAN B, et al. A novel icephobic strategy: the fabrication of biomimetic coupling micropatterns of superwetting surface[J]. Advanced Materials Interfaces, 2019, 6(19): 1900864. DOI:10.1002/admi.201900864 |
| [67] |
MILLES S, SOLDERA M, VOISIAT B, et al. Fabrication of superhydrophobic and ice-repellent surfaces on pure aluminium using single and multiscaled periodic textures[J]. Scientific Reports, 2019, 9(1): 13944. DOI:10.1038/s41598-019-49615-x |
| [68] |
LIU Y, LI X, YAN Y, et al. Anti-icing performance of superhydrophobic aluminum alloy surface and its rebounding mechanism of droplet under super-cold conditions[J]. Surface & Coatings Technology, 2017, 331: 7-14. |
| [69] |
连峰, 王增勇, 张会臣. 疏水/超疏水船用铝合金表面制备及其耐久性[J]. 材料工程, 2015, 43(1): 49-53. LIAN F, WANG Z Y, ZHANG H C. Preparation of hydrophobic/superhydrophobic warship aluminium alloy surface and its durability[J]. Journal of Materials Engineering, 2015, 43(1): 49-53. |
| [70] |
QIN Y, LI Y, ZHANG D, et al. Wettability, durability and corrosion properties of slippery laser-textured aluminum alloy surface under water impact[J]. Surface & Coatings Technology, 2020, 394: 125856. |
| [71] |
RUAN M, WANG J W, LIU Q L, et al. Superhydrophobic and anti-icing properties of sol-gel prepared alumina coatings[J]. Russian Journal of Non-Ferrous Metals, 2016, 57(6): 638-645. DOI:10.3103/S1067821216060122 |
| [72] |
弯艳玲, 奚传文, 董宾, 等. 微纳复合沟槽形铝合金表面的结冰性能[J]. 中国表面工程, 2018, 31(4): 81-87. WAN Y L, XI C W, DONG B, et al. Icing performance of micro-nano composite grooves on aluminum alloy surface[J]. China Surface Engineering, 2018, 31(4): 81-87. |
| [73] |
GUO P, ZHENG Y, WEN M, et al. Icephobic/anti-icing properties of micro/nanostructured surfaces[J]. Advanced Materials, 2012, 24(19): 2642-2648. DOI:10.1002/adma.201104412 |
| [74] |
SHEN Y, XIE X, XIE Y, et al. Statistically understanding roles of nanostructure features in interfacial ice nucleation for enhancing the icing delay performance[J]. Physical Chemistry Chemical Physics, 2019, 21(36): 19785-19794. DOI:10.1039/C9CP04103F |
| [75] |
SHEN Y, JIN M, WU X, et al. Understanding the frosting and defrosting mechanism on the superhydrophobic surfaces with hierarchical structures for enhancing anti-frosting performance[J]. Applied Thermal Engineering, 2019, 156: 111-118. DOI:10.1016/j.applthermaleng.2019.04.052 |
| [76] |
PAPADOPOULOS P, MAMMEN L, DENG X, et al. How superhydrophobicity breaks down[J]. Proceedings of the National Academy of Science, 2013, 110(9): 3254-3258. DOI:10.1073/pnas.1218673110 |
| [77] |
RYKACZEWSKI K, ANAND S, SUBRAMANYAM S B, et al. Mechanism of frost formation on lubricant-impregnated surfaces[J]. Langmuir, 2013, 29(17): 5230-5238. DOI:10.1021/la400801s |
| [78] |
KULINICH S A, FARHADI S, NOSE K, et al. Superhydrophobic surfaces: are they really ice-repellent?[J]. Langmuir, 2011, 27(1): 25-29. DOI:10.1021/la104277q |
| [79] |
RØNNEBERG S, HE J, ZHANG Z. The need for standards in low ice adhesion surface research: a critical review[J]. Journal of Adhesion Science and Technology, 2019, 34(3): 1-29. |
| [80] |
RØNNEBERG S, LAFORTE C, VOLAT C, et al. The effect of ice type on ice adhesion[J]. AIP Advances, 2019, 9(5): 055304. DOI:10.1063/1.5086242 |
| [81] |
GOLOVIN K, DHYANI A, THOULESS M D, et al. Low-interfacial toughness materials for effective large-scale deicing[J]. Science, 2019, 364(6438): 371-375. DOI:10.1126/science.aav1266 |
| [82] |
MAKKONEN L. Ice adhesion—theory, measurements and countermeasures[J]. Journal of Adhesion Science and Technology, 2012, 26(4/5): 413-445. |
| [83] |
LIU Y, ZHANG Z, HU H, et al. An experimental study to characterize a surface treated with a novel laser surface texturing technique: water repellency and reduced ice adhesion[J]. Surface & Coatings Technology, 2019, 374: 634-644. |
| [84] |
MEMON H, LIU J, De FOCATIIS D S A, et al. Intrinsic dependence of ice adhesion strength on surface roughness[J]. Surface & Coatings Technology, 2020, 385: 125382. |
| [85] |
LI X, ZHAO Y, LI H, et al. Preparation and icephobic properties of polymethyltrifluoropropylsiloxane-polyacrylate block copolymers[J]. Applied Surface Science, 2014, 316: 222-231. DOI:10.1016/j.apsusc.2014.07.097 |
| [86] |
FU Q, WU X, KUMAR D, et al. Development of sol-gel icephobic coatings: effect of surface roughness and surface energy[J]. ACS Applied Materials & Interfaces, 2014, 6(23): 20685-20692. |
 2021, Vol. 49
2021, Vol. 49


