文章信息
- 韩超越, 候冰娜, 郑泽邻, 徐文静, 沈惠玲, 李征征
- HAN Chao-yue, HOU Bing-na, ZHENG Ze-lin, XU Wen-jing, SHEN Hui-ling, LI Zheng-zheng
- 功能高分子材料的研究进展
- Research progress in functional polymer materials
- 材料工程, 2021, 49(6): 55-65
- Journal of Materials Engineering, 2021, 49(6): 55-65.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2020.000487
-
文章历史
- 收稿日期: 2020-05-28
- 修订日期: 2021-01-19
2. 南京霖厚环保科技有限公司, 南京 210001
2. Nanjing Linhou Environmental Protection Technology Co., Ltd., Nanjing 210001, China
功能高分子材料是20世纪60年代发展起来的一种新材料,通过在天然或合成高分子主链和侧链上接枝反应性功能基团,使其具有新的诸如催化性、导电性、光敏性、导磁性、生物活性等特殊功能的一类新型高分子[1-3]。功能高分子材料对物质、能量、信息具有传输、转换或贮存的作用,又被称为特种高分子材料或者精细高分子材料[4-6]。如图 1所示,功能高分子材料分为反应型功能高分子材料、光功能高分子材料、电磁功能高分子材料、生物医用功能高分子材料等几大类[7-11],因其具有催化性、导电性、光敏性、导磁性、生物活性等特殊的功能而备受人们关注。目前对功能高分子材料的研究主要集中在其结构和性能之间的关系上,通过优化功能高分子材料合成方法,开发出新型功能高分子材料,不断扩展其应用领域。

|
图 1 功能高分子材料种类 Fig. 1 Types of functional polymer materials |
功能高分子材料具有种类多样、产量少、专用性强等特点,此外,与其他功能材料相比,功能高分子材料还具有质量轻、结构配方可设计性强等特点,因此可以广泛满足各个应用领域的要求[12-14]。通常合成的功能高分子材料不单单只有一种功能,比如通过高分子改性能够制备具有导热性、导磁性和导电性的多功能高分子材料[15-18]。此外,不同功能之间可以相互转换和交叉,比如具有光电效应的材料能够实现光功能和电功能的可逆转换[19-21]。随着对功能高分子材料研究的日益成熟,功能高分子材料逐渐形成了光电磁高分子信息材料和医用高分子材料两个主要研究领域,并且正在往高功能化、多功能化和智能化方向发展。因此许多新型智能高分子材料被陆续开发出来,比如分子自组装材料、形状记忆材料、智能水凝胶和纳米复合材料等[22-27]。
功能高分子材料的高性能和专用性的特点使其广泛应用于各个领域。例如,吸附分离功能高分子材料主要包括离子交换树脂和吸附树脂,通过离子交换能够达到分离提纯的目的,在天然产物分离纯化、血液净化治疗等领域有极大的应用[28-30]。电磁功能高分子材料具有导电性和导磁性,可以制成导电聚合物膜、电磁制动器、电磁波干扰屏蔽材料和抗静电材料等[31-33]。光功能高分子材料具有对光吸收、储存和转化的功能,可以制成光纤、有机玻璃眼镜、光致变色材料等[34-35]。生物医用功能高分子材料在当代医学领域具有更广泛的应用,被用作人工器官、药物递送载体等[36-39]。本文介绍了几类不同的功能高分子材料的性能及其应用。
1 功能高分子材料发展现状 1.1 反应型功能高分子材料反应型功能高分子材料包括高分子试剂和高分子催化剂,通过将反应活性中心或催化性中心接枝到高分子链上,实现小分子试剂或催化剂的高分子化[40]。常见的高分子试剂根据化学活性可分为氧化试剂、还原试剂、烷基化试剂、酰基化试剂、卤代试剂和固相合成试剂等[41-43]。高分子催化剂包括用于酸碱催化的离子交换树脂、过渡金属络合物催化剂、相转移催化剂和固定化酶等[44-46]。反应型高分子材料要求具有高反应活性、高选择性和专一性,主要用于化学合成和化学反应。
高分子试剂和催化剂不仅具有小分子的反应性能和催化性能,还具有多孔性、高选择性和化学稳定性等特殊性能,拓展了化学试剂和催化剂的应用范围[47]。固相合成试剂是高分子试剂中应用非常广泛的一种试剂,多用于合成多肽、核苷酸、寡糖等生物活性大分子[48]。固相合成法是利用连接在高分子载体上的活性官能团与小分子试剂进行连续多步反应,得到最终产物后再通过水解脱除载体的合成方法[49]。Li等[50]通过两步固相/溶液法合成了海绵环肽(phakellistatin 15),反应以线性八肽为原料,选用2-氯三苯甲基氯树脂为固相载体,乙酸/三氟乙醇/二氯甲烷裂解树脂为保护试剂,通过溶液法实现线性八肽的环化。phakellistatin 15是一种天然的富含脯氨酸的环八肽,具有很好的抗菌性、抗病毒性、酶抑制性和抗肿瘤性,在生物医学中具有很高的应用价值,但phakellistatin 15很难从自然界中提取且产率极低,因此需要通过化学反应合成。这种两步固相/溶液法相比其他合成方法提高了合成产率(35.46%),缩短了反应时间(1 h)。
酶是一种能加速生化反应和化学反应的大分子生物催化剂,在温和的反应条件下具有较高的催化效率和底物特异性,被广泛应用于各个领域,但其稳定性较差,很容易变性失活,基于这点通常在不影响酶的活性的前提下对酶进行固定化。Cirillo等[51]以聚(乙二醇)二甲基丙烯酸酯为交联剂,N-异丙基丙烯酰胺为单体制备了具有温敏性的水凝胶,并通过自由基聚合将胃蛋白酶共价固定在水凝胶上,合成了具有酶催化活性的温敏水凝胶。通过控制温度能够调节胃蛋白酶的活性,当温度高于水凝胶的临界溶液温度(45 ℃)时,水凝胶未表现出催化活性,当温度降低到室温时水凝胶恢复酶催化活性。此外,通过热稳定性实验发现,高温下水凝胶仍保留酶的催化活性,即高温不会使酶失活,因此,能够通过改变温度来可逆地获得酶催化作用。Feng等[52]基于碳二亚胺偶联技术实现了脂肪酶与多壁碳纳米管(MWCNT)的共价结合。首先用HNO3纯化MWCNT,并用H2SO4和HNO3氧化以引入羧基。经1-乙基-(3-二甲基氨基丙基)碳二亚胺盐酸盐(EDC)和N-羟基琥珀酰亚胺(NHS)活化后,脂肪酶通过酰基酰胺键被固定在羧基化的MWCNT上。固定后的脂肪酶显示出较低的温度依赖性和较高的分离效率。研究表明固定化脂肪酶的二级结构发生了变化,这可能导致酶活性的降低,但其在有机溶剂中的催化活性却得到了显著提高。
1.2 光功能高分子材料光功能高分子材料是指能够对光能进行吸收存储、传输、转换的一类高分子材料[53]。光功能高分子材料主要包括光稳定剂、光敏涂料、荧光剂、光转化材料、光致变色材料和光导材料等[54-55]。光功能高分子材料在生产生活中的应用非常广泛,比如光导纤维、太阳能、集成电路和光电池等[56-57]。
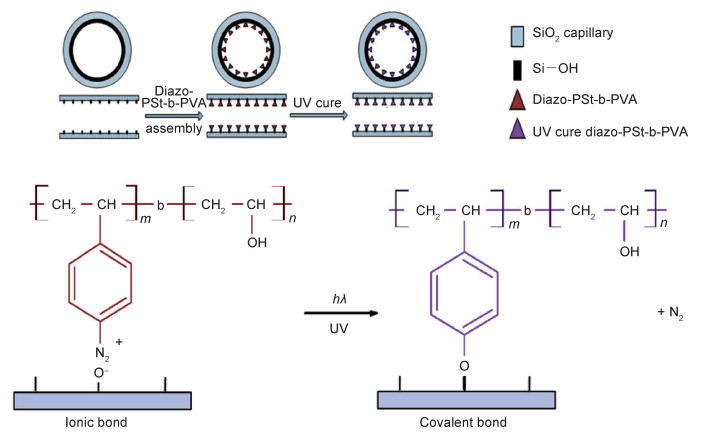
Lee等[58]通过偶联缩合反应合成了两种基于二苯甲酮的荧光聚合物pCzBP和pAcBP,并将2-乙基己基侧链接枝在pCzBP和pAcBP聚合物链段上,从而使pCzBP和pAcBP具有较好的溶解度,易于溶于常见的有机溶剂,例如甲苯、氯仿和氯苯等。pCzBP和pAcBP能够发射TADF,其外部量子效率(ηext=(9.3±0.9)%)比常规荧光聚合物的理论极限外部量子效率(ηext < 5%)要高。此外,pCzBP和pAcBP还表现出良好的热稳定性和固态下的非晶态结构,能够用作有机发光二极管。Yu等[59]通过将光敏涂料重氮聚乙烯醇/苯乙烯涂覆在毛细管的内表面上,制备了一种能用于蛋白质分离的毛细管。如图 2所示,涂有光敏涂料的毛细管经紫外线照射后,重氮基团发生光化学反应将离子键转变为共价键,共价连接的涂层会抑制蛋白质在毛细管内表面的吸附,实现了核糖核酸酶A、溶菌酶和牛血清白蛋白的基线蛋白质分离。与非共价涂覆或裸露的毛细管相比,共价连接的毛细管涂层具有更高的电泳分离性能以及出色的可重复性和稳定性,是一种环保、安全的蛋白质分离技术。
光致变色材料是一种应用很广泛的光功能高分子材料,光致变色材料在光学数据存储和光学交换方面具有潜在的应用价值。Tanino等[60]合成了一种基于偶氮苯的新型光致变色非晶态材料,能够应用在防伪、装饰、显示、摄影、信息存储等领域。该新型光致变色非晶态材料自身能够形成均匀的非晶态膜而无需聚合物黏合剂,并表现出玻璃化转变现象。研究发现,合成的光致变色材料在非晶态膜和溶液中均容易表现出光致变色特性,其作为非晶薄膜的反式-顺式光异构化的量子产率要低于溶液中的量子产率。此外,基于材料结构的不同,光致变色非晶态膜的顺式-反式热异构化的表观速率常数也与溶液中的稍有不同。Mutoh等[61]以聚(甲基丙烯酸甲酯)和聚(丙烯酸丁酯)组成的三嵌段聚合物(PMMA-b-PBA)为基体,3H-萘并吡喃为光致变色分子合成了一种能够快速光致变色的聚合物膜。3H-萘并吡喃通过光致变色反应能够改变其分子结构和偶极矩,而3H-萘并吡喃2和10取代位上的溴、苯基和芘基等大取代基会引起空间和静电排斥,从而提高其褪色速度,极大地提高了3H-萘并吡喃光致变色反应的效率。此外,PMMA-b-PBA同时具有刚性链和柔性链,刚性链提高了聚合物膜的力学性能,柔性链则为光致变色反应中的结构变化提供了足够的自由体积。
1.3 电功能高分子材料导电功能高分子材料按组成可分为结构型导电高分子和复合型导电高分子两类[62]。结构型导电高分子依靠自身提供的导电载流子导电,这类高分子经掺杂后导电率能够大幅度提升,而复合型导电高分子需要通过添加炭黑、金属粉、箔等来实现导电[63-64]。结构型导电高分子材料主要有聚乙炔、线型聚苯、氮硫高聚物、聚酮酞菁等,但单一的结构型导电高分子材料电导率不高,在实际应用中,需要掺杂电子受体或电子给体[65-66]。复合型导电高分子材料包括导电塑料、导电橡胶、导电涂料和导电薄膜等[67-68],在复合型导电高分子中,高分子充当黏合剂的角色,本身不具有导电性。复合型导电高分子材料制备简单,实用性强,其主要应用于发光二极管、电致发光和电磁屏蔽器等领域中[69-70]。
Aydin等[71]设计了一种用于检测人血清和唾液中白细胞介素的生物传感器。通过将四臂星形聚甲基丙烯酸缩水甘油酯(SPGMA)、导电剂炭黑和黏合剂聚偏氟乙烯(PVDF)混合制备出均质的导电复合浆料,然后将其涂布在ITO电极表面上,白细胞介素抗体与星形聚合物的环氧基团共价连接,制备了具有电化学性能的生物传感器。导电复合材料提高了该生物传感器的灵敏性,降低了白细胞介素的检测限,此外,该生物传感器的制备过程简单,并且与酶联免疫吸附测定试剂盒兼容。
Aycan等[72]制备了一种海藻酸钠/明胶/透明质酸/氧化石墨烯导电复合聚合物薄膜(SAlg/Gel/HA/RGO),其中SAlg/Gel/HA作为聚合物网络,RGO通过分子间的范德华相互作用和氢键作用均匀分布在聚合物网络中。RGO作为电活性材料,为SAlg/Gel/HA/RGO聚合物薄膜提供了导电性。RGO的掺入提高了聚合物薄膜的电导率,但随着RGO添加量的增大电导率逐渐降低,RGO分子也会发生团聚,研究表明RGO最佳添加量的体积分数为10%。此外,SAlg/Gel/HA/RGO聚合物膜相比纯聚合物膜具有更优异的力学性能,RGO的加入提高了聚合物薄膜的弹性模量、拉伸强度和断裂伸长率。研究发现,SAlg/Gel/HA/RGO聚合物膜还具有良好的透气性和药物缓释性能,能够很好地释放布洛芬抗炎药,提高了SAlg/Gel/HA/RGO膜对伤口的愈合效果。SAlg/Gel/HA/RGO膜在伤口敷料方面具有很好的应用前景。
1.4 生物医用功能高分子材料生物医用高分子材料是一种用于生理系统疾病的诊断和治疗,修复或替换生物体组织器官的高分子材料,包括医用高分子和药用高分子两大类[73-74]。生物医用高分子材料被广泛应用于人工器官、药物释放、生物组织工程等领域[75-77]。由于生物医用高分子材料直接应用于人体,因此要求其要无毒无害,其次要有良好的生物相容性,此外根据使用场合的不同对材料还有其他的特殊要求[78-79]。
Meng等[80]通过将果胶(QP)和蒙脱土(MMT)简单共混制备了QP-MMT杂化膜,该膜具有良好的药物缓释性能。果胶具有良好的生物相容性,易降解,被广泛用作药物载体,但果胶在体内易被溶解且药物释放速度较快,因此作者通过混入MMT来减缓药物的释放速度,达到药物缓释的目的。选用5-氟尿嘧啶为释放药物,研究了QP-MMT杂化膜的体外药物缓释性能,发现最佳膜表现出较高的药物包封率(36.50%)和载药率(80.30%)。此外,MMT的存在确实改善了果胶的缓释性能,QP10-MMT0.1杂化膜的药物累积释放率在前0.5 h均在20%左右,药物持续释放时间超过了8 h。此外,研究人员进行了细胞毒性实验,证明了QP-MMT杂化膜具有良好的生物相容性。
Bai等[81]等通过阳离子聚(芴-亚苯基)衍生物(PFP-NMe3+)与葫芦[7]尿嘧啶(CB[7])合成了一种超分子络合物(PFP-NMe3+/CB[7]),能够用于快速原位检测和鉴别多种病原体。真菌和细菌的细胞壁均带有负电荷,具有两亲结构的PFP-NMe3+可通过静电和疏水相互作用与病原体带负电荷的细胞壁结合。CB[7]能够掩埋PFP-NMe3+侧链上的烷基从而减弱PFP-NMe3+与病原体表面的疏水相互作用,当向PFP-NMe3+/CB[7]络合物中添加金刚烷胺(AD)后,由于AD会与CB[7]形成更加稳定的CB[7]/AD络合物,从而使PFP-NMe3+从PFP-NMe3+/CB[7]络合物中释放。因此,PFP-NMe3+/CB[7]络合物在添加AD前后会与病原体表现出不同的相互作用方式。通过计算添加AD前后被PFP-NMe3+/CB[7]染色的病原体荧光强度的变化,实现对病原体简单、快速区分。研究发现,添加AD后,革兰氏阳性菌的荧光强度显著增加,而革兰氏阴性菌和真菌的荧光强度则明显减少,表明PFP-NMe3+/CB[7]络合物能够成功检测出一个样品中的多种病原体。而且该检测方法快速、简单,仅需2 h即可对各种病原体进行分层,并且不需要特定的生物标记或细胞标记。Ishihara等[82]合成了2-甲基丙烯酰氧乙基磷酰胆碱聚合物(MPC),具有良好的血液相容性和生物相容性,可用于制造人工器官等。MPC聚合物相比于传统材料具有很好的血液相容性,在没有使用抗凝剂的情况下与血液接触也可以有效抑制血液的凝固。将人全血分别涂覆在玻璃和MPC聚合物上,发现血液在玻璃上的凝结时间为8.4 min,而在MPC聚合物上的凝结时间显著增加为28 min,这是因为MPC聚合物能够有效抑制血浆蛋白的减少和吸附蛋白的变性。此外,MPC聚合物具有良好的抗蛋白质吸附和细胞黏附性能,因此被广泛应用于临床器官的表面修饰和生物医学设备。
2 功能高分子材料发展趋势 2.1 环境降解高分子材料近年来,高分子材料的发展非常迅速,应用也日益广泛,但高分子材料在自然环境中很难分解,造成大量的白色污染,这就使发展可降解高分子材料成为必然趋势[83]。降解高分子材料分为光降解高分子材料和生物降解高分子材料两类[84-86]。高分子材料通过引入感光基团或添加光敏剂来制备光降解高分子材料,在光的作用下光降解高分子材料的聚合物链断裂,分子量降低[87]。光降解高分子材料主要用于包装材料和农膜,但其应用条件苛刻、价格较贵,因此生物降解高分子材料在近几年更受关注[88-89]。生物降解高分子材料是指通过生物酶作用或微生物化学作用能够发生降解的高分子。生物降解高分子材料包括淀粉、纤维素、甲壳素、透明质酸等天然高分子材料和乳酸、聚己内酯等合成高分子材料[90-92]。生物降解高分子材料具有质量轻、价格便宜以及易降解等特点而被广泛应用于生物工程和医用降解高分子材料等领域。
Nayanathara等[93]通过苯乙烯(St)与天然肉桂油和合成肉桂醛(Cin)之间的自由基共聚合制备了两种苯乙烯-肉桂醛聚合物薄膜(St-co-Cin),研究了在室外风化条件下和紫外线辐射条件下薄膜的光降解作用。研究发现,紫外线照射下苯乙烯-肉桂醛共聚物的羰基吸收光后诱发Norrish Ⅰ和Norrish Ⅱ降解反应,反应产生的自由基会通过自氧化作用继续降解。在室外风化条件下,苯乙烯-肉桂醛共聚物的失重率远高于聚苯乙烯均聚物的失重率,而且在风化10天后共聚物开始变色,而聚苯乙烯均聚物没有发生变色。此外,随着肉桂醛含量的增加,苯乙烯-肉桂醛共聚物薄膜的失重率增高。在各时间间隔内,通过合成肉桂醛制备的共聚物的失重率均高于与天然肉桂油合成的共聚物的失重率。因此,制备的苯乙烯-肉桂醛共聚物薄膜在药用包装以及食品包装工业中具有潜在的应用。
Sevostyanov等[94]合成了一种聚乳酸-乙醇酸薄膜(PLGA),具有生物降解性和药物缓释性能。研究发现,合成的PLGA膜具有一定的力学性能,相对伸长率为25%~165%,拉伸强度为20~55 MPa。此外,PLGA膜是可生物降解的,每天的降解率为0.5%~1.0%。PLGA薄膜能够持续和定向释放生物大分子,特别是具有高溶栓活性的链激酶分子,经PLGA膜释放的链激酶分子约90%,保持其活性,链激酶的释放速率为每天0.01~0.07 mg/cm2。当将PLGA薄膜样品植入动物体内两个月后,仅在组织中检测到了微量的PLGA,并且在术后没有并发症,表明合成的PLGA膜对细胞没有任何毒性作用。因此,合成的新型可生物降解的PLGA聚合物膜具有一定的力学性能,并且能够持续定向地释放药物,在生物医药领域具有巨大的应用潜能。
2.2 形状记忆高分子材料形状记忆材料是在改变并固定其形状后,通过改变外界条件(温度、pH、电场力等)能够使其恢复初始形状的材料[95]。形状记忆高分子材料根据引起形状记忆效应条件的不同分为热致感应型、电致感应型、光致感应型和化学感应型,其中热致感应型形状记忆高分子材料应用最为广泛[96-98]。形状记忆高分子材料具有质量轻、形变量大、成型容易等优点,被用于医疗、包装、建筑等领域[99]。
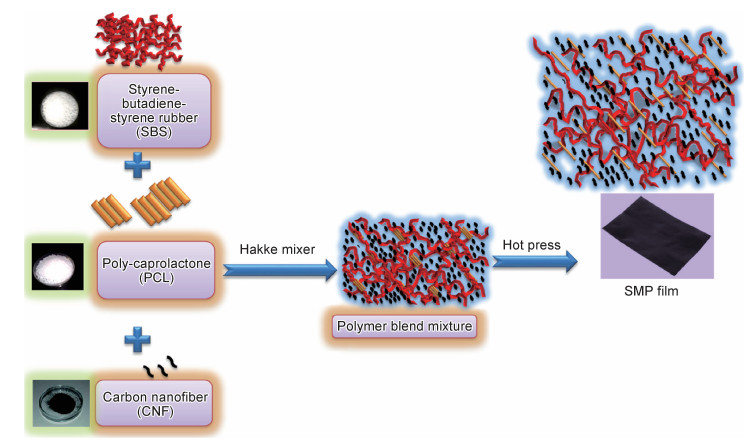
Gopinath等[100]通过熔融共混法制备了具有热响应形状记忆功能的高分子纳米复合材料,该材料由聚己内酯(PCL)、聚苯乙烯-丁二烯-苯乙烯嵌段共聚物(SBS)和碳纳米纤维(CNF)共混合成(如图 3所示)。其中,PCL因为熔点较低(60 ℃)充当开关聚合物,提供热响应形状记忆性能。此外,PCL和SBS的混合能够提供材料更好的弹性和柔韧性,而CNF会增强材料的导热性和导电性。研究表明,PCL含量会影响纳米复合材料的形状记忆性能,当PCL含量低于12.5%时,材料不具有形状记忆功能。SBS弹性体有助于材料形状的恢复,此外合成的纳米复合材料还具有较好的热稳定性。Capiel等[101]通过自由基聚合反应由月桂酸、油酸、甲基丙烯酸缩水甘油酯和苯乙烯制备了一种形状记忆材料。该材料高度半透明且不溶于常见的有机溶剂和水,此外还具有适度的交联密度。研究还发现,高的交联密度有利于材料的形状恢复。Ma等[102]选用星形聚乳酸和低聚苯胺合成了一种兼有形状记忆功能和导电功能的共聚物(ESMP)。低聚苯胺的加入增强了ESMP共聚物的拉伸应力,使所得的形状记忆共聚物具有很高的机械强度。另外,ESMP共聚物还具有优异的形状记忆能力和生物降解性。
高分子水凝胶是一种由亲水性高分子通过化学或物理交联而形成的具有三维网络结构的聚合物,能够吸收并保持大量的水[103]。高分子水凝胶具有与天然组织相似的微环境,都有很高的含水量(最高达99%),在生物医药领域有广泛的应用,如伤口敷料、隐形眼镜、组织工程和药物递送领域[104-106]。当高分子水凝胶所处的环境(温度、pH、离子浓度、光、磁场、电场和化学物质等)发生变化时,高分子凝胶的结构或体积相变也会产生相应的改变,这种水凝胶被称为智能水凝胶。基于智能高分子水凝胶的刺激响应性,其被广泛应用于传感器、驱动器、药物载体和生物催化等领域[107-109]。
Zheng等[110]合成了一种基于壳聚糖的温敏性可注射水凝胶,通过将壳聚糖溶液(CS)与β-甘油磷酸盐(β-GP)物理混合制得,此外凝胶中还封装了光热材料(MoS2/Bi2S3-PEG,MBP纳米片)和抗肿瘤药物(阿霉素,DOX),从而实现了结肠癌的光热和化学疗法的结合。当温度高于37 ℃时,由于CS和β-GP之间的氢键、静电相互作用和疏水相互作用增强,水凝胶会发生溶胶-凝胶转变。当CS/MBP/DOX溶液注入人体后会发生凝胶化,实现DOX和MBP纳米片的封装从而阻止其进入血液循环,提高药物治疗的安全性。此外,CS基水凝胶还具有很好的抗菌能力,进一步增强了载药凝胶的安全性。研究表明,CS/MBP/DOX水凝胶的凝胶温度可以通过近红外激光辐照来控制,因此DOX的释放是可控的,该水凝胶能够有效地用于肿瘤治疗。Solomevich等[111]研究了基于葡聚糖磷酸酯(DP)水凝胶的脯氨酸药物递送系统,以及其用于局部癌症治疗的可能性。研究表明,制得的DP水凝胶对pH敏感,并且具有生物降解性。此外,还研究了水凝胶的药物释放性能,发现药物的释放量取决于外部介质的pH值,并且随着DP水凝胶中磷基含量的增加而减少。研究还发现,DP水凝胶具有体外细胞毒性和体内抗肿瘤活性。这证明DP水凝胶可以用作药物控释的载体,在治疗胃肠道恶性肿瘤方面具有巨大的潜力。
3 结束语本文介绍了反应型功能高分子材料、光功能高分子材料、电功能高分子材料和生物医用功能高分子材料等几类常用的功能高分子,对其分类、作用机理以及应用等方面进行了简要阐述。随着功能高分子材料的蓬勃发展和实际应用的需求,功能高分子材料向着环境友好型和智能化发展,因此本文还着重介绍了环境降解高分子材料、形状记忆高分子材料和智能高分子水凝胶。
多功能高分子材料由于其功能的多样化,在生产生活中具有更加广泛的应用。因此,功能高分子材料近年来逐渐向着多功能化方向发展,电磁材料、导电材料、光热材料等相继出现。此外,随着科学技术的不断进步,研究人员对高分子结构与性能之间关系的研究也逐渐深入,制备出越来越多的具有特殊功能的新型功能高分子材料,比如生物高分子材料、隐身高分子材料等,进一步扩大了功能高分子材料的范围。基于对高分子材料应用方面的更高要求,为克服高分子材料强度低、易老化、使用寿命短等缺点,兼有传统功能(电功能、光功能等)和特殊功能(自修复、形状记忆功能等)的功能高分子材料将是未来材料的研究方向。相信随着对高分子材料结构的深入研究,兼有两种或以上功能的高分子材料将进一步被扩展,有望应用于航空航天、医疗、食品、工业等各个领域。
| [1] |
HONG M, LI Y G, LI B X, et al. Synthesis of polyethylene containing allene groups: a simple and efficient route to functional polyethylene[J]. Macromolecular Rapid Communications, 2012, 33(11): 998-1002. DOI:10.1002/marc.201100855 |
| [2] |
BUCHMEISER M R. Polymeric monolithic materials: syntheses, properties, functionalization and applications[J]. Polymer, 2007, 48(8): 2187-2198. DOI:10.1016/j.polymer.2007.02.045 |
| [3] |
BECKER G, WURM F R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups[J]. Chemical Society Reviews, 2018, 47(20): 7739-7782. DOI:10.1039/C8CS00531A |
| [4] |
ZHAO F, BAE J, ZHOU X, et al. Nanostructured functional hydrogels as an emerging platform for advanced energy technologies[J]. Advanced Materials, 2018, 30(48): 1-16. |
| [5] |
DAS S, CHAKRABORTY P, MONDAL S, et al. Enhancement of energy storage and photoresponse properties of folic acid-polyaniline hybrid hydrogel by in situ growth of Ag nanoparticles[J]. ACS Applied Materials and Interfaces, 2016, 8(41): 28055-28067. DOI:10.1021/acsami.6b09468 |
| [6] |
HVILSTED S, RAMANUJAM P S. Polymer scaffolds bearing azobenzene-potential for optical information storage[J]. Chinese Journal of Polymer Science, 2001, 19(2): 147-153. |
| [7] |
BOLIVAR J M, ROCHA-MARTIN J, MATEO C, et al. Coating of soluble and immobilized enzymes with ionic polymers: full stabilization of the quaternary structure of multimeric enzymes[J]. Biomacromolecules, 2009, 10(4): 742-747. DOI:10.1021/bm801162e |
| [8] |
DORING A, BIRNBAUM W, KUCKLING D. Responsive hydrogels-structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science[J]. Chemical Society Reviews, 2013, 42(17): 7391-7420. DOI:10.1039/c3cs60031a |
| [9] |
MCDONALD M B, HAMMOND P T. Efficient transport networks in a dual electron/lithium-conducting polymeric composite for electrochemical applications[J]. ACS Applied Materials and Interfaces, 2018, 10(18): 15681-15690. DOI:10.1021/acsami.8b01519 |
| [10] |
NAGAHAMA K, TAKAHASHI A, OHYA Y. Biodegradable polymers exhibiting temperature-responsive sol-gel transition as injectable biomedical materials[J]. Reactive and Functional Polymers, 2013, 73(7): 979-985. DOI:10.1016/j.reactfunctpolym.2012.11.003 |
| [11] |
GUO J, WANG Q, JIN J, et al. Analysis of structure and electrochemistry of selenium-containing conductive polymer materials for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 2016, 163(5): 654-659. DOI:10.1149/2.0661605jes |
| [12] |
TONHAUSER C, FREY H. A road less traveled to functional polymers: epoxide termination in living carbanionic polymer synthesis[J]. Macromolecular Rapid Communications, 2010, 31(22): 1938-1947. DOI:10.1002/marc.201000353 |
| [13] |
IKKALA O, BRIN G T. Functional materials based on self-assembly of polymeric supramolecules[J]. Science, 2002, 295(5564): 2407-2409. DOI:10.1126/science.1067794 |
| [14] |
SEDO J, SAIZ-POSEU J, BUSQUE F, et al. Catechol-based biomimetic functional materials[J]. Advanced Materials, 2013, 25(5): 653-701. DOI:10.1002/adma.201202343 |
| [15] |
FOX R T, WANI V, HOWARD K E, et al. Conductive polymer composite materials and their utility in electromagnetic shielding applications[J]. Journal of Applied Polymer Science, 2007, 107(4): 2558-2566. |
| [16] |
SAHA N, SEDLARIK V, SAHA P, et al. Electromagnetic properties of aluminosilicate-filled polymer composites of poly(vinyl alcohol)-poly(vinyl pyrrolidone)[J]. Polymer Composites, 2005, 26(6): 739-744. DOI:10.1002/pc.20146 |
| [17] |
SUSLYAEV V I, KUZNETSOV V L, ZHURAVLEV V A, et al. An investigation of electromagnetic response of composite polymer materials containing carbon nanostructures within the range of frequencies 10 MHz-1.1 THz[J]. Russian Physics Journal, 2013, 55(8): 970-976. DOI:10.1007/s11182-013-9909-7 |
| [18] |
LI S, WANG X, HU R, et al. Near-infrared absorbing conjugated polymer dots as highly-effective photothermal materials for in vivo cancer therapy[J]. Chemistry of Materials, 2016, 28(23): 8669-8675. DOI:10.1021/acs.chemmater.6b03738 |
| [19] |
FAN Y, LIN B, SUN Y, et al. A new method to make polymers with flexible main chains and photoelectric pendants for organic semiconductors[J]. Polymer Chemistry, 2013, 4(15): 4245-4255. DOI:10.1039/c3py00454f |
| [20] |
LI Y, HU Y, ZHAO Y, et al. An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics[J]. Advanced Materials, 2011, 23(6): 776-780. DOI:10.1002/adma.201003819 |
| [21] |
VANNIKOV A V, GRISHINA A D, RYCHWALSKI R W. Photoelectric, nonlinear optical and photorefractive properties of polymer/carbon nanotube composites[J]. Carbon, 2011, 49(1): 311-319. DOI:10.1016/j.carbon.2010.09.028 |
| [22] |
WU J, ZHANG Q, WANG J, et al. A self-assembly route to porous polyaniline/reduced graphene oxide composite materials with molecular-level uniformity for high-performance supercapacitors[J]. Energy and Environmental Science, 2018, 11(5): 1280-1286. DOI:10.1039/C8EE00078F |
| [23] |
LU Y, LIU L, FOO W, et al. Self-assembled polymer layers of linear polyethylenimine for enhancing electrochromic cycling stability[J]. Journal of Materials Chemistry C, 2013, 1(23): 3651-3654. DOI:10.1039/c3tc30447g |
| [24] |
LUO H, LIU Y, YU Z, et al. Novel biodegradable shape memory material based on partial inclusion complex formation between alpha-cyclodextrin and poly(epsilon-caprolactone)[J]. Biomacromolecules, 2008, 9(10): 2573-2577. DOI:10.1021/bm8004726 |
| [25] |
BAI X, BAO Z, BI S, et al. Chitosan-based thermo/pH double sensitive hydrogel for controlled drug delivery[J]. Macromolecular Bioscience, 2018, 18(3): 1700305. DOI:10.1002/mabi.201700305 |
| [26] |
SHIM W S, YOO J S, BAE Y H, et al. Novel injectable pH and temperature sensitive block copolymer hydrogel[J]. Biomacromolecules, 2005, 6(6): 2930-2934. DOI:10.1021/bm050521k |
| [27] |
BEAUJUGE P M, FRECHET J M J. Molecular design and ordering effects in π-functional materials for transistor and solar cell applications[J]. Journal of the American Chemical Society, 2011, 133(50): 20009-20029. DOI:10.1021/ja2073643 |
| [28] |
WANG W, LI X, YUAN S, et al. Effect of resin charged functional group, porosity, and chemical matrix on the long-term pharmaceutical removal mechanism by conventional ion exchange resins[J]. Chemosphere, 2016, 160: 71-79. DOI:10.1016/j.chemosphere.2016.06.073 |
| [29] |
WANG J, LI H B, SHUANG C D, et al. Effect of pore structure on adsorption behavior of ibuprofen by magnetic anion exchange resins[J]. Microporous and Mesoporous Materials, 2015, 210: 94-100. DOI:10.1016/j.micromeso.2015.02.026 |
| [30] |
JIANG M, YANG W B, ZHANG Z W, et al. Adsorption of three pharmaceuticals on two magnetic ion-exchange resins[J]. Journal of Environmental Sciences, 2015, 31(5): 226-234. |
| [31] |
HU C C, CHANG S S, LIANG N Y. Preparation and characterization of carbon black/polybutylene terephthalate/polyethylene terephthalate antistatic fiber with sheath-core structure[J]. The Journal of the Textile Institute, 2016, 107(8): 976-984. |
| [32] |
VOLKOV V P, ZELENETSKY A N, SHEVCHENKO V G, et al. Synthesis and properties of electromagnetic wave shielding polymer materials with low flammability[J]. Journal of Applied Polymer Science, 2010, 116(5): 2775-2782. |
| [33] |
NAYAK L, KHASSTGIR D, CHAKI T K. A mechanistic study on electromagnetic shielding effectiveness of polysulfone/carbon nanofibers nanocomposites[J]. Journal of Materials Science, 2013, 48(4): 1492-1502. DOI:10.1007/s10853-012-6904-2 |
| [34] |
SAVA I, SACARESCU L, STOICA I, et al. Photochromic properties of polyimide and polysiloxane azopolymers[J]. Polymer International, 2009, 58(2): 163-170. DOI:10.1002/pi.2508 |
| [35] |
ERCOLE F, MALIC N, HARRISSON S, et al. Photochromic polymer conjugates: the importance of macromolecular architecture in controlling switching speed within a polymer matrix[J]. Macromolecules, 2010, 43(1): 249-261. DOI:10.1021/ma901830b |
| [36] |
AHMED T, MARCAL H, LAWLESS M, et al. Polyhydroxybutyrate and its copolymer with polyhydroxyvalerate as biomaterials: influence on progression of stem cell cycle[J]. Biomacromolecules, 2010, 11(10): 2707-2715. DOI:10.1021/bm1007579 |
| [37] |
LIU K H, CHEN S Y, LIU D M, et al. Self-assembled hollow nanocapsule from amphiphatic carboxymethyl-hexanoyl chitosan as drug carrier[J]. Macromolecules, 2008, 41(17): 6511-6516. DOI:10.1021/ma8002399 |
| [38] |
ZHANG Z, CHEN L B, GAO J, et al. Preparation of poly(sebacic anhydride) and polylactic acid pills used as drug carrier for levofloxacin controlled release[J]. Journal of Polymer Engineering, 2013, 33(7): 659-664. DOI:10.1515/polyeng-2013-0071 |
| [39] |
QIU F, WANG D, ZHU Q, et al. Real-time monitoring of anticancer drug release with highly fluorescent star-conjugated copolymer as a drug carrier[J]. Biomacromolecules, 2014, 15(4): 1355-1364. DOI:10.1021/bm401891c |
| [40] |
QIN T, CORNELLA J, LI C, et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents[J]. Science, 2016, 352(6287): 801-805. DOI:10.1126/science.aaf6123 |
| [41] |
BAUER A M, RAMEY E E, OBERLE K G, et al. Cross-linked poly(4-vinylpyridine-N-oxide) as a polymer-supported oxygen atom transfer reagent[J]. Tetrahedron Letters, 2019, 60(43): 151193. DOI:10.1016/j.tetlet.2019.151193 |
| [42] |
LI H, PANG Z B, JIAO Z F, et al. Synthesis and application of a microgel-supported acylating reagent by coupled ring-opening metathesis polymerization and activators re-generated by electron transfer for atom transfer radical polymerization[J]. Journal of Combinatorial Chemistry, 2010, 12(2): 255-259. DOI:10.1021/cc900162w |
| [43] |
BHATTACHARYYA S, RANA S, GOODING O W, et al. Polymer-supported triacetoxyborohydride: a novel reagent of choice for reductive amination[J]. Tetrahedron Letters, 2003, 44(27): 4957-4960. DOI:10.1016/S0040-4039(03)01167-5 |
| [44] |
REN Z, LYU Y, SONG X, et al. Dual-ionically bound single-site rhodium on porous ionic polymer rivals commercial methanol carbonylation catalysts[J]. Advanced Materials, 2019, 31(50): 1904976. DOI:10.1002/adma.201904976 |
| [45] |
CHEN X, ZHU H, WANG W, et al. Multifunctional single-site catalysts for alkoxycarbonylation of terminal alkynes[J]. ChemSusChem, 2016, 9(17): 2451-2459. DOI:10.1002/cssc.201600660 |
| [46] |
DINGWALL L D, LEE A F, LYNAM J M, et al. Bifunctional organorhodium solid acid catalysts for methanol carbonylation[J]. ACS Catalysis, 2012, 2(7): 1368-1376. DOI:10.1021/cs3000528 |
| [47] |
LANGSTON J A, COLBY R H, CHUNG T C M. Synthesis and characterization of long chain branched isotactic polypropylene via metallocene catalyst and T-reagent[J]. Macromolecules, 2007, 40(8): 2712-2720. DOI:10.1021/ma062111+ |
| [48] |
JAD Y E, ACOSTA G A, GOVENDER T, et al. Green solid-phase peptide synthesis-2.2-methytetrahydrofuran and ethyl acetate for solid-phase peptide synthesis under green conditions[J]. ACS Sustainable Chemistry and Engineering, 2016, 4(12): 6809-6814. DOI:10.1021/acssuschemeng.6b01765 |
| [49] |
FULOPOVA V, KRCHNAK V. Solid-phase synthesis of trisubstituted 2, 5-dihydrobenzo[J]. ACS Combinatorial Science, 2014, 16(8): 412-420. DOI:10.1021/co500084k |
| [50] |
LI Y L, WU M H, CHANG Q, et al. Solid-phase synthesis of cyclic octapeptide phakellistatin 15[J]. Chemistry of Natural Compounds, 2019, 55(3): 520-524. DOI:10.1007/s10600-019-02729-0 |
| [51] |
CIRILLO G, NICOLETTA F P, CURCIO M, et al. Enzyme immobilization on smart polymers: catalysis on demand[J]. Reactive and Functional Polymers, 2014, 83: 62-69. DOI:10.1016/j.reactfunctpolym.2014.07.010 |
| [52] |
TAN H, FENG W, JI P. Lipase immobilized on magnetic multi-walled carbon nanotubes[J]. Bioresource Technology, 2012, 115: 172-176. DOI:10.1016/j.biortech.2011.10.066 |
| [53] |
RICHARD B S, SHALAV A. The role of polymers in the luminescence conversion of sunlight for enhanced solar cell performance[J]. Synthetic Metals, 2005, 154(1/3): 61-64. |
| [54] |
DIAZ S A, MENENDEZ G O, ETCHEHON M H, et al. Photoswitchable water-soluble quantum dots: pcFRET based on amphiphilic photochromic polymer coating[J]. ACS Nano, 2011, 5(4): 2795-2805. DOI:10.1021/nn103243c |
| [55] |
WU B, LEI Y, XIAO Y, et al. A bio-inspired and biomass-derived healable photochromic material induced by hierarchical structural design[J]. Macromolecular Materials and Engineering, 2020, 305(1): 1900539. DOI:10.1002/mame.201900539 |
| [56] |
CUI Y, YAO H F, HONG L, et al. Achieving over 15% efficiency in organic photovoltaic cells via copolymer design[J]. Advanced Materials, 2019, 31(14): 1808356. DOI:10.1002/adma.201808356 |
| [57] |
XU Y, CHEN L, GUO Z, et al. Light-emitting conjugated polymers with microporous network architecture: interweaving scaffold promotes electronic conjugation, facilitates exciton migration, and improves luminescence[J]. Journal of the American Chemical Society, 2011, 133(44): 17622-17625. DOI:10.1021/ja208284t |
| [58] |
LEE S Y, YASUDA T, KOMIYAMA H, et al. Thermally activated delayed fluorescence polymers for efficient solution-processed organic light-emitting diodes[J]. Advanced Materials, 2016, 28(21): 4019-4024. DOI:10.1002/adma.201505026 |
| [59] |
YU B, PENG Q, USMAN M, et al. Preparation of photosensitive diazotized poly (vinyl alcohol-b-styrene) covalent capillary coatings for capillary electrophoresis separation of proteins[J]. Journal of Chromatography A, 2019, 1593: 174-182. DOI:10.1016/j.chroma.2019.02.004 |
| [60] |
TANINO T, YOSHIKAWA S, UJIKE T, et al. Creation of azobenzene-based photochromic amorphous molecular materials-synthesis, glass-forming properties, and photochromic response[J]. Journal of Materials Chemistry, 2007, 17(47): 4953-4963. DOI:10.1039/b711542c |
| [61] |
MUTOH K, KOBAYASHI Y, ABE J, et al. Efficient coloration and decoloration reactions of fast photochromic 3H-naphthopyrans in PMMA-b-PBA block copolymer[J]. Dyes and Pigments, 2017, 137: 307-311. DOI:10.1016/j.dyepig.2016.11.004 |
| [62] |
CAO Q, HUANG A, ZHANG W, et al. Progress in conductive properties of carbon-based doped polymer[J]. Polymer Materials Science and Engineering, 2012, 28(4): 177-181. |
| [63] |
FELLER J F, LINOSSIER I, LEVESQUE G. Conductive polymer composites (CPCs): comparison of electrical properties of poly(ethylene-co-ethyl acrylate)-carbon black with poly(butylene terephthalate)/poly(ethylene-co-ethyl acrylate)-carbon black[J]. Polymers for Advanced Technologies, 2010, 13(10/12): 714-724. |
| [64] |
STARY Z, KRUCKEL J. Conductive polymer composites with carbonic fillers: shear induced electrical behaviour[J]. Polymer, 2018, 139: 52-59. DOI:10.1016/j.polymer.2018.02.008 |
| [65] |
HONORATO A M B, KHALID M, DAI Q, et al. Nitrogen and sulfur co-doped fibrous-like carbon electrocatalyst derived from conductive polymers for highly active oxygen reduction catalysis[J]. Synthetic Metals, 2020, 264: 116383. DOI:10.1016/j.synthmet.2020.116383 |
| [66] |
MATYSIAK W, TANSKI T, SMOK W, et al. Effect of conductive polymers on the optical properties of electrospun polyacrylonitryle nanofibers filled by polypyrrole, polythiophene and polyaniline[J]. Applied Surface Science, 2019, 509: 145068. |
| [67] |
CHEN Z, HSU P C, LOPEZ J, et al. Fast and reversible thermoresponsive polymer switching materials for safer batteries[J]. Nature Energy, 2016, 1(1): 15009. DOI:10.1038/nenergy.2015.9 |
| [68] |
GREWAL M S, TANAKA M, KAWAKAMI H. Fabrication and characterizations of soft and flexible poly(dimethylsiloxane)-incorporated network polymer electrolyte membranes[J]. Polymer, 2020, 186: 122045. DOI:10.1016/j.polymer.2019.122045 |
| [69] |
YU W C, ZHANG G Q, LIU Y H, et al. Selective electromagnetic interference shielding performance and superior mechanical strength of conductive polymer composites with oriented segregated conductive networks[J]. Chemical Engineering Journal, 2019, 373: 556-564. DOI:10.1016/j.cej.2019.05.074 |
| [70] |
HAN H J, ZHOU D D, REN Q Y, et al. High-performance all-polymer dielectric and electrical energy storagematerials containing conjugated segment and multi-fluorinated pendants[J]. European Polymer Journal, 2020, 122: 109376. DOI:10.1016/j.eurpolymj.2019.109376 |
| [71] |
AYDIN M, AYDIN E B, SEZGINTURK M K. A highly selective electrochemical immunosensor based on conductive carbon black and star PGMA polymer composite material for IL-8 biomarker detection in human serum and saliva[J]. Biosensors and Bioelectronics, 2018, 117: 720-728. DOI:10.1016/j.bios.2018.07.010 |
| [72] |
AYCAN D, SELMI B, KELEL E, et al. Conductive polymeric film loaded with ibuprofen as a wound dressing material[J]. European Polymer Journal, 2019, 121: 109308. DOI:10.1016/j.eurpolymj.2019.109308 |
| [73] |
TEO A J T, MISHRA A, PARK I, et al. Polymeric biomaterials for medical implants and devices[J]. ACS Biomaterials Science and Engineering, 2016, 2(4): 454-472. DOI:10.1021/acsbiomaterials.5b00429 |
| [74] |
PAN Z, DING J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine[J]. Interface Focus, 2012, 2(3): 366-377. DOI:10.1098/rsfs.2011.0123 |
| [75] |
BITAR R, COOLS P, DE GEYTER N, et al. Acrylic acid plasma polymerization for biomedical use[J]. Applied Surface Science, 2018, 448: 168-185. DOI:10.1016/j.apsusc.2018.04.129 |
| [76] |
BOMBIN A D J, NICHOLAS J D, MCCARTHY H O B. Electrospinning of natural polymers for the production of nanofibres for wound healing applications[J]. Materials Science and Engineering: C, 2020, 114: 110994. DOI:10.1016/j.msec.2020.110994 |
| [77] |
ZHANG A, JUNG K, Li A, et al. Recent advances in stimuli-responsive polymer systems for remotely controlled drug release[J]. Progress in Polymer Science, 2019, 99: 101164. DOI:10.1016/j.progpolymsci.2019.101164 |
| [78] |
UMAPATHI R, KHAN I, AYCAN J A P, et al. Unravelling the interactions between biomedical thermoresponsive polymer and biocompatible ionic liquids[J]. Journal of Molecular Liquids, 2020, 300: 112362. DOI:10.1016/j.molliq.2019.112362 |
| [79] |
TIAN H, TANG Z, ZHUANG X, et al. Biodegradable synthetic polymers: preparation, functionalization and biomedical application[J]. Progress in Polymer Science, 2012, 37(2): 237-280. DOI:10.1016/j.progpolymsci.2011.06.004 |
| [80] |
MENG Y J, WANG S Y, GUO Z W, et al. Design and preparation of quaternized pectin-montmorillonite hybrid film for sustained drug release[J]. International Journal of Biological Macromolecules Volume, 2020, 154: 413-420. DOI:10.1016/j.ijbiomac.2020.03.140 |
| [81] |
BAI H, CHEN H, HU R, et al. Supramolecular conjugated polymer materials for in-situ pathogen detection[J]. ACS Applied Materials and Interfaces, 2016, 8(46): 31550-31557. DOI:10.1021/acsami.6b09807 |
| [82] |
ISHIHARA K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs[J]. Science and Technology of Advanced Materials, 2000, 1(3): 131-138. DOI:10.1016/S1468-6996(00)00012-7 |
| [83] |
YIN G Z, YANG X M. Biodegradable polymers: a cure for the planet, but a long way to go[J]. Journal of Polymer Research, 2020, 27(2): 1-14. DOI:10.1007/s10965-020-2004-1 |
| [84] |
ISOLA C, SIEVERDING H L, RAGHUNATHAN R, et al. Life cycle assessment of photodegradable polymeric material derived from renewable bioresources[J]. Journal of Cleaner Production, 2016, 142: 2935-2944. |
| [85] |
DOS SANTOS P P, FLORES S H, DE OLIVEIRA RIOS A, et al. Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules[J]. Trends in Food Science and Technology, 2016, 53: 23-33. DOI:10.1016/j.tifs.2016.05.005 |
| [86] |
GRUBER M F, SCHULTE L, NDONI S. Nanoporous materials modified with biodegradable polymers as models for drug delivery applications[J]. Journal of Colloid and Interface Science, 2013, 395(1): 58-63. |
| [87] |
FAIRBANKS B D, SINGH S P, BOWMAN C N, et al. Photodegradable, photoadaptable hydrogels via radical-mediated disulfide fragmentation reaction[J]. Macromolecules, 2011, 44(8): 2444-2450. DOI:10.1021/ma200202w |
| [88] |
ULERY B D, NAIR L S, LAURENCIN C T. Biomedical applications of biodegradable polymers[J]. Journal of Polymer Science Part B, 2011, 49(12): 832-864. DOI:10.1002/polb.22259 |
| [89] |
ADHIKARI R, GUNATILLAKE P A, GRIFFITHS I, et al. Biodegradable injectable polyurethanes: synthesis and evaluation for orthopaedic applications[J]. Biomaterials, 2008, 29(28): 3762-3770. DOI:10.1016/j.biomaterials.2008.06.021 |
| [90] |
AHMAD N, AHMAD R, ALAM M A, et al. Daunorubicin oral bioavailability enhancement by surface coated natural biodegradable macromolecule chitosan based polymeric nanoparticles[J]. International Journal of Biological Macromolecules, 2019, 128: 825-838. DOI:10.1016/j.ijbiomac.2019.01.142 |
| [91] |
DU Z, SU Y Z, QU Y Y, et al. A mechanically robust, biodegradable and high performance cellulose gel membrane as gel polymer electrolyte of lithium-ion battery[J]. Electrochimica Acta, 2019, 299: 19-26. DOI:10.1016/j.electacta.2018.12.173 |
| [92] |
JOO Y S, CHA J R, GONG M S, et al. Biodegradable shape-memory polymers using polycaprolactone and isosorbide based polyurethane blends[J]. Materials Science and Engineering C, 2018, 91: 426-435. DOI:10.1016/j.msec.2018.05.063 |
| [93] |
NAYANATHARA U, KOTTEGODA N, PERERA I C, et al. Synthesis, photodegradable and antibacterial properties of polystyrene-cinnamaldehyde copolymer film[J]. Polymer Degradation and Stability, 2018, 155: 195-207. DOI:10.1016/j.polymdegradstab.2018.07.021 |
| [94] |
SEVOSTYANOV M A, BAIKIN A S, SERGIENKO K V, et al. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase[J]. Reactive and Functional Polymers, 2020, 150: 104550. DOI:10.1016/j.reactfunctpolym.2020.104550 |
| [95] |
LENG J, LAN X, LIU Y, et al. Shape-memory polymers and their composites: stimulus methods and applications[J]. Progress in Materials Science, 2011, 56(7): 1077-1135. DOI:10.1016/j.pmatsci.2011.03.001 |
| [96] |
MONROE M B B, EASLEY A D, GRANT K, et al. Multifunctional shape-memory polymer foams with bio-inspired antimicrobials[J]. ChemPhysChem, 2018, 19(16): 1999-2008. DOI:10.1002/cphc.201701015 |
| [97] |
WANG X, GUO X, YE J, et al. Freestanding 3D mesostructures, functional devices, and shape-programmable systems based on mechanically induced assembly with shape memory polymers[J]. Advanced Materials, 2019, 31(2): 1805615. DOI:10.1002/adma.201805615 |
| [98] |
WANG Z D, SONG W B, KE L L, et al. Shape memory polymer composite structures with two-way shape memory effects[J]. Materials Letters, 2012, 89: 216-218. DOI:10.1016/j.matlet.2012.08.112 |
| [99] |
BEHL M, RAZZAQ M Y, LENDLEIN A. Multifunctional shape-memory polymers[J]. Advanced Materials, 2010, 22(31): 3388-3410. DOI:10.1002/adma.200904447 |
| [100] |
GOPINATH S, ADARSH N N, NAIR P R, et al. One-way thermo-responsive shape memory polymer nanocomposite derived from polycaprolactone and polystyrene-block-polybutadiene-blockpolystyrene packed with carbon nanofiber[J]. Materials Today Communications, 2020, 22: 100802. DOI:10.1016/j.mtcomm.2019.100802 |
| [101] |
CAPIEL G, MARCOVICH N E, MOSIEWICKI M A. Shape memory polymer networks based on methacrylated fatty acids[J]. European Polymer Journal, 2019, 116: 321-329. DOI:10.1016/j.eurpolymj.2019.04.023 |
| [102] |
XIE M, WANG L, GE J, et al. Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering[J]. ACS Applied Materials and Interfaces, 2015, 7(12): 6772-6781. DOI:10.1021/acsami.5b00191 |
| [103] |
KIM K, BAE B, KANG Y J, et al. Natural polypeptide-based supramolecular nanogels for stable noncovalent encapsulation[J]. Biomacromolecules, 2013, 14(10): 3515-3522. DOI:10.1021/bm400846h |
| [104] |
WANG Q, MYNAR J L, YOSHIDA M, et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder[J]. Nature, 2010, 463(7279): 339-343. DOI:10.1038/nature08693 |
| [105] |
董建成, 葛孝栋, 王清清, 等. 阳离子光敏抗菌型水凝胶的制备及性能表征[J]. 材料工程, 2019, 47(2): 60-65. DONG J C, GE X D, WANG Q Q, et al. Preparation and property characterization of cationic photoantimicrobial hydrogel[J]. Journal of Materials Engineering, 2019, 47(2): 60-65. |
| [106] |
SELIKTAR D. Designing cell-compatible hydrogels for biomedical applications[J]. Science, 2012, 336(6085): 1124-1128. DOI:10.1126/science.1214804 |
| [107] |
AGARWAL A K, DONG L, BEEBE D J, et al. Autonomously-triggered microfluidic cooling using thermo-responsive hydrogels[J]. Lab on A Chip, 2007, 7(3): 310-315. DOI:10.1039/b617767k |
| [108] |
GUO J, PAN S, YIN X, et al. pH-sensitive keratin-based polymer hydrogel and its controllable drug-release behavior[J]. Journal of Applied Polymer Science, 2014, 132(9): 41572-41579. |
| [109] |
LIU P, GUO B, WANG S, et al. A thermo-responsive and self-healing liposome-in-hydrogel system as an antitubercular drug carrier for localized bone tuberculosis therapy[J]. International Journal of Pharmaceutics, 2019, 558: 101-109. DOI:10.1016/j.ijpharm.2018.12.083 |
| [110] |
ZHENG Y T, WANG W F, ZHAO J L, et al. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer[J]. Carbohydrate Polymers, 2019, 222: 115039. DOI:10.1016/j.carbpol.2019.115039 |
| [111] |
SOLOMEVICH S O, BYCHKOVSKY P M, YURKSHTOVICH T L, et al. Biodegradable pH-sensitive prospidine-loaded dextran phosphate based hydrogels for local tumor therapy[J]. Carbohydrate Polymers, 2019, 226: 115308. DOI:10.1016/j.carbpol.2019.115308 |
 2021, Vol. 49
2021, Vol. 49