文章信息
- 葛玉杰, 吴姣, 何志强, 王国华, 刘金香
- GE Yu-jie, WU Jiao, HE Zhi-qiang, WANG Guo-hua, LIU Jin-xiang
- g-C3N4基异质耦合光催化剂制备及在环境污染物去除领域的研究进展
- Research progress in preparation and application of g-C3N4-based heterogeneous photocatalyst for environmental pollutant removal
- 材料工程, 2021, 49(4): 23-33
- Journal of Materials Engineering, 2021, 49(4): 23-33.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2020.000285
-
文章历史
- 收稿日期: 2020-03-30
- 修订日期: 2020-10-13
2. 南华大学 污染控制与资源化技术湖南省重点实验室, 湖南 衡阳 421001
2. Hunan Provincial Key Laboratory of Pollution Control and Resources Technology, University of South China, Hengyang 421001, Hunan, China
工业的快速发展使得环境污染不断加剧,污染物的排放对生态环境和人类健康造成严重的危害[1-2]。近年来,具有安全环保、价格低廉、无二次污染等优点的光催化技术在能源以及环境修复领域得到广泛关注[3-4]。而光催化剂作为光催化技术的核心,通过将太阳能转化为化学能从而实现清洁能源转化以及对环境污染物降解是解决能源短缺以及环境修复问题的有效途径。只具有紫外光响应范围(在太阳光中的占比小于5%)的TiO2, ZnO等传统光催化剂对太阳光利用率较低[5],开发高效稳定、廉价的可见光响应光催化剂的研究成为光催化技术发展的关键。
石墨相氮化碳(g-C3N4)是一种非金属半导体材料,通常是通过热聚合法将尿素、氰胺类等富含氮、碳前驱体高温煅烧制得。根据密度泛函理论(DFT)的计算, g-C3N4的基本单体结构是由稳定的三嗪结构单元和七嗪结构单元组成,因此具有较高的热稳定性和化学稳定性[6];g-C3N4具有合适的带隙结构(窄带隙2.7 eV)、性能可调控性强等优点[7-9],在近年来被广泛应用于光催化水解制氢、降解有机污染物、抗菌等研究[10-12]。但是纯g-C3N4结晶度低、激子结合能较高,不利于光生载流子的分离从而导致光催化性能降低;此外,较低的比表面积、可见光吸收能力较弱等缺点使g-C3N4在光催化的研究与应用中面临了巨大挑战[13-14]。研究者利用模板辅助法、液相剥离、元素掺杂、与其他材料异质耦合等策略不断改善g-C3N4的光催化性能[15],而在众多的优化方法中,异质耦合被认为是抑制g-C3N4光生电子-空穴复合过快、拓宽可见光吸收范围,提高光催化性能最有效的方法[16]。本文综述了以g-C3N4为基础的异质耦合光催化剂的催化机理、种类等,并总结g-C3N4基光催化剂在污染物处理方面的研究进展。
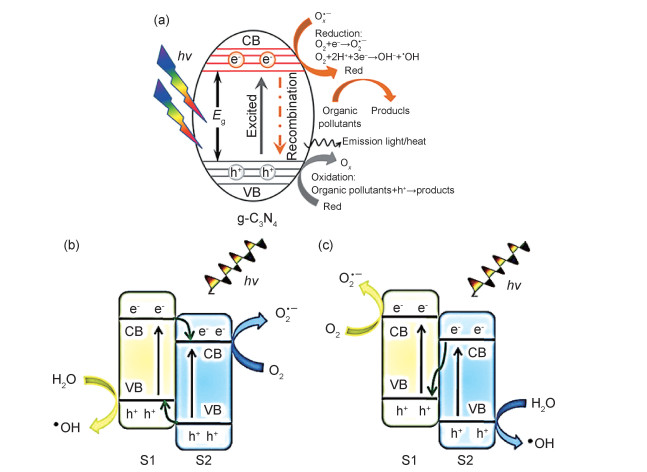
1 g-C3N4基异质耦合的光催化机理 1.1 g-C3N4的光催化机理利用g-C3N4作为半导体催化剂去除污染物(图 1(a))可以概括为三个阶段[17]:(1)光捕获阶段;(2)电荷激发、转移阶段;(3)表面反应阶段。通过吸收太阳光中的可见光来激发g-C3N4,在价带(VB)产生的光生电子(e-)跃迁至导带(CB)而光生空穴(h+)滞留在价带(VB),最终e-与h+分别在催化剂价带(VB)、导带(CB)与污染物发生氧化、还原反应并达到去除污染物的目的。但是纯g-C3N4的光激发电子-空穴极易复合导致其产率较低,严重影响了g-C3N4材料的光催化性能。
g-C3N4与不同材料通过异质耦合形成多元光催化剂,即在材料的接触界面形成异质结。半导体/g-C3N4型异质结是目前光催化剂的研究热点之一:既利用不同半导体之间所具有的带隙能级差实现光生电子-空穴的有效分离从而提高光催化性能。根据g-C3N4与不同半导体之间能带结构的不同,研究者提出了g-C3N4/半导体异质结构中两种潜在的双电荷转移途径,即Ⅱ型异质结和直接Z型结构机制[18-19]。对于Ⅱ型异质结(图 1(b)),半导体S1上的光激发电子转移至半导体S2的价带上并发生还原反应,而光生空穴则由S2转移至S1的导带上并发生氧化反应,以此电荷转移方式来延长光生电子与空穴的复合时间;Z型异质结(图 1(c))是近几年研究较为广泛的一种半导体异质耦合方式:其中半导体S1比半导体S2具有更高的价带、导带位置,两种半导体上的光生电子-空穴被激发分离后,半导体S2中的光生电子与S1中导带上的空穴复合,在S2中导带上的空穴与S1中的价带上的电子继续参与氧化还原反应。
贵金属/g-C3N4异质结是通过贵金属纳米粒子半导体在g-C3N4表面沉积所形成的。一方面,贵金属纳米粒子所在局域表面受局部电磁场影响下形成等离子体共振(LSPR),加速g-C3N4光催化剂导带、价带的形成[20];另一方面,具有量子约束效应和较低费米能级水平的贵金属纳米粒子形成肖特基势垒来促进光生电子-空穴的分离,将电子转移至具有更低负电位的金属纳米离子上来提高量子产率,从而阻止光生电子-空穴的重新复合[21]。
碳材料/g-C3N4异质耦合型催化剂的催化机理与前者类似,其催化原理是利用碳材料的高电子储存容量以及较好的电子传导性提高光生电荷从g-C3N4催化剂向液-固界面的转移效率,增强光催化剂的可见光吸收能力[22]。此外,较大的比表面积和孔隙能够提供足量的吸附位点来增强对污染物的吸附能力[23]。
2 g-C3N4基二元异质耦合光催化剂 2.1 贵金属/g-C3N4利用贵金属(Ag, Au, Pt等)在g-C3N4表面进行沉积,不仅可以优化催化剂的表面性质,更能改变整个催化体系的电子分布从而有效提高其光催化性能[20-21, 24]。Qin等[20]利用化学还原法将Ag纳米粒子均匀沉积在g-C3N4表面,Ag粒子通过LSPR效应增加g-C3N4对可见光吸收并减小光生电子-空穴的复合效率,与纯g-C3N4相比,催化活性提高了3.6倍。适量的金属纳米粒子沉积还能解决单一g-C3N4比表面积较小的问题,Fu等[21]成功将Au纳米粒子固定在g-C3N4表面,与纯g-C3N4相比,Au/g-C3N4的比表面积(58.9 m2/g)是原始g-C3N4比表面积(34.8 m2/g)的1.7倍,通过增大比表面积增加活性催化位点,能够显著提高光催化性能。
拓宽光催化材料的可见光响应范围和提高光生载流子的分离效率是提高光催化性能的两个重要影响因素[25]。Yu等[24]利用硼氢化钠辅助浸渍与还原制备得到Pt/g-C3N4复合材料,紫外-可见光图谱(UV-Vis)显示g-C3N4的光吸收带在450 nm附近,而经过Pt沉积之后的复合催化剂吸收带发生明显红移,说明复合催化剂对可见光的吸收范围不断拓宽。光致发光光谱(PL)发射谱图对催化剂光生载流子的分离效率检测结果表明Pt的沉积有效抑制了光生载流子间的复合。相比于其他金属单质,贵金属具有较高的活性和耐久性、重复利用性好等优点,能有效提高光催化性能;缺点是价格昂贵,难以在实际工程中广泛应用。
2.2 过渡金属半导体/g-C3N4近几年,以Ti, Zn, Mo, Cd, V, W等过渡金属元素形成的氧化物、硫化物为代表的半导体材料成为与g-C3N4异质耦合的研究热点。
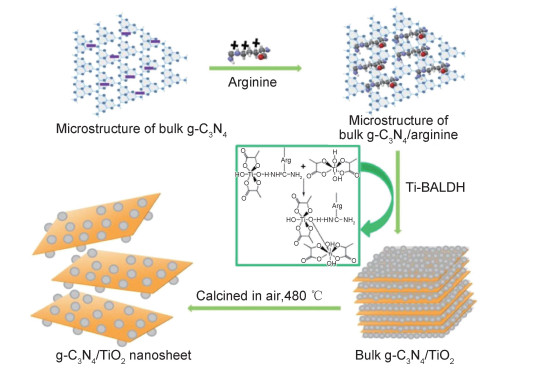
2.2.1 过渡金属氧化物/g-C3N4二氧化钛(TiO2)作为传统的光催化材料具有可利用性强、价格低廉、化学稳定性高等优点,但其能带边缘位于紫外光响应范围,对太阳光利用率较低[26]。利用TiO2与g-C3N4进行异质耦合能减少g-C3N4光生电子的复合,同时可以增强TiO2在可见光下的光催化活性。Tong等[27]通过一种绿色仿生矿化合成法(图 2),利用生物分子在温和条件下催化钛前驱体水解缩合制备TiO2/g-C3N4异质耦合光催化剂:带正电荷的精氨酸分子被吸附到带负电荷的g-C3N4上,精氨酸所带羟基、羧基与g-C3N4所带氨基形成氢键后,将带负电的钛前驱体(Ti-BALDH)引入系统使非晶态TiO2原位生长,位于g-C3N4上的精氨酸分子诱导非晶态TiO2凝聚,随后逐层热氧化刻蚀使TiO2结晶并改善g-C3N4分层。利用该方法合成的TiO2/g-C3N4光催化剂在可见光照射下50 min几乎可以降解水溶液中所有的罗丹明B(Rh-B)。氧化锌(ZnO)同样是一种宽带隙(3.37 eV)紫外光响应型的光催化剂,由于g-C3N4的电阻较大导致光激发电子转移受阻,而具有较大比表面积的纳米ZnO有助于电子沿其轴向转移,为光激发电子的转移提供更多通道[28]。Zhong等[29]利用煅烧法合成的g-C3N4/ZnO纳米棒异质催化剂具有良好的稳定性,该催化剂对多种有机污染物(亚甲基蓝(MB), Rh-B等)均具有较高的降解率,通过自由基捕获实验以及电子自旋共振实验(ESR)结果得出催化剂降解有机染料的主要活性物质为·O2-和·OH。
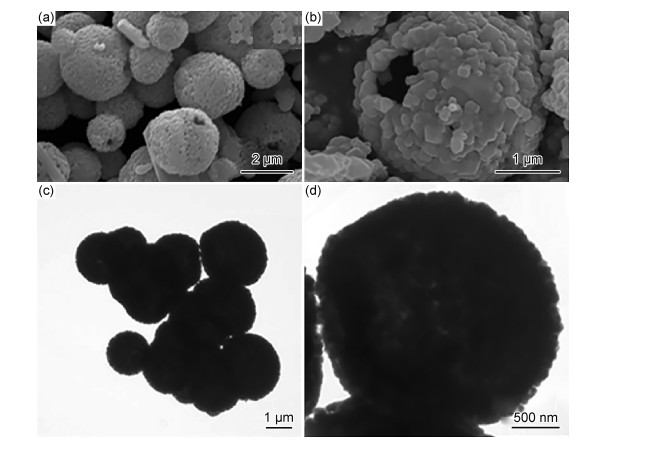
除利用紫外光响应型光催化剂与g-C3N4异质耦合外,表 1总结了其他具有较低带隙可见光响应过渡金属氧化物与g-C3N4的异质耦合形式,它们通常与g-C3N4具有匹配的带隙结构,通过异质耦合作用形成复合光催化剂能有效解决光生载流子复合过快的问题。氧化钨(WO3)是一种具有较高的化学惰性与光稳定性的可见光响应型光催化剂,其VB位置与TiO2非常接近从而具有较强的氧化能力,但较低CB水平不仅还原能力弱且会导致光生电子-空穴的复合[30]。考虑到其带隙结构与g-C3N4高度匹配,Xiao等[31]利用原位水解聚合法制备WO3/g-C3N4复合中空微球(CHMS), 如图 3所示,与传统的材料表面负载法不同,空心结构可延长入射光的捕获时间从而提高可见光利用率;WO3与g-C3N4之间形成较大的接触面积有利于提高光生电子-空穴的分离效率。该催化剂对盐酸四环素和头孢噻肟钠具有较高的降解效率,在60 min时的去除量分别为9.64,6.90 mg/g。
| Material | Synthesis strategy | Application | Reference |
| Fe3O4/g-C3 N4 | Hydrothermal method | Porous Fe3O4/g-C3N4 nanospheres showed considerable photocatalytic activity, and exhibited excellent reusability and magnetic properties. | [35] |
| CuO/g-C3N4 | Thermal condensation | CuO/g-C3N4 exhibited the highest catalytic activity, which was more than 100% greater than the parent g-C3N4. | [36] |
| ZrO2/g-C3N4 | Calcination and hydrothermal method | The activity of the ZrO2/g-C3N4 for photodegradation of MB is much higher than that of either pure g-C3N4 or ZrO2. | [37] |
| MoO3/g-C3N4 | Mixing and annealing process | The incorporation of the inactive MoO3 significantly enhances the overall activity, and the Rh-B can be completely degraded within 10-15 min. | [38] |
| CoFe2O4/g-C3N4 | Calcination | The degradation rate of MB reached 97.3% at 3 h. | [39] |
| MnWO4/g-C3N4 | Hydrothermal method | The degradation rate of Rh-B is 73%. | [40] |
具有较强可见光吸收能力的氧化钒(V2O5)能带边缘与g-C3N4相匹配,可形成直接固态Z型异质结光催化剂。Dadigala等[32]通过原位浸渍法合成棒状V2O5/g-C3N4 1D/2D型复合催化剂,与传统粒状V2O5相比,比表面积较大的棒状结构能够为界面的电荷分离提供较大的接触面积。尖晶石铁氧体(MFe2O4,M=Mn2+, Zn2+等)是一类具有较强可见光响应的磁性半导体催化剂[33],Palanivel等[34]利用溶胶-凝胶法制备了NiFe2O4/g-C3N4复合催化剂,g-C3N4的加入能有效抑制纳米NiFe2O4粒子的团聚现象;与单一催化剂相比,NiFe2O4/g-C3N4对MB和Rh-B的降解效率均达到99%,在多次循环使用后通过外加磁场可进行有效回收,显著提高了催化剂的利用效率。磁性可回收纳米催化剂具有较高的研究价值和实际应用价值。
2.2.2 过渡金属硫化物/g-C3N4以CdS, MoS2, WS2为代表的过渡金属硫化物在近几年被广泛应用于半导体/g-C3N4异质耦合光催化剂的构建中。CdS的带隙较窄(2.42 eV)且具有560 nm以上的可见光吸收范围,良好的光生载流子迁移率使其作为一种可见光响应的催化剂受到广泛关注[41]。将g-C3N4与CdS异质耦合也是一种提高g-C3N4光催化活性的有效策略,Wang等[14]通过密度泛函理论(DFT)计算了g-C3N4/CdS异质结的带隙结构,g-C3N4与CdS之间形成的Ⅱ型异质结通过界面内置电场促进光生载流子的有效分离,提高光催化效率;通过计算得出的异质结带隙变窄,这更有利于提高催化剂对可见光的吸收能力。Jiang等[42]利用沉淀法合成g-C3N4/CdS催化剂,片状g-C3N4的加入抑制了CdS量子点在水中的团聚和光腐蚀现象,该催化剂对MB的降解速率在180 min内达90.45%,其催化活性较g-C3N4与CdS明显增强。
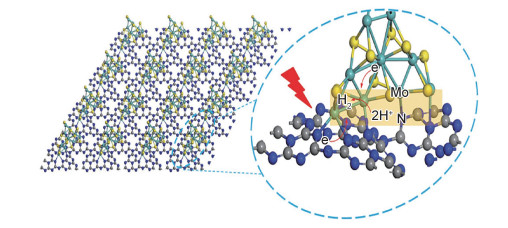
g-C3N4与半导体异质耦合可以分为0D/2D, 1D/2D, 2D/2D三种耦合形式,其中2D/2D异质结的接触面积最大,可有效促进电荷的转移;此外,2D层状结构缩短了光生载流子的扩散距离和时间,提高了光生电子的分离效率[43]。二维层状MoS2具有合适的带隙结构和价、导带位置,是构建2D/2D异质耦合光催化剂的理想材料。Gao等[44]利用浸渍-煅烧法将不同量的MoS2与g-C3N4充分混合并在不同温度下煅烧,进一步巩固异质结界面。MoS2与g-C3N4相似的微观结构能减少不同晶格参数之间的失配,有效促进MoS2在g-C3N4表面上的生长。经过与MoS2异质耦合后的g-C3N4对可见光吸收范围从486 nm拓宽至504 nm,投加量为2.0 g/L的MoS2/g-C3N4在120 min内对Rh-B的降解效率达到99.8%。Bian等[45]以硫脲与层状MoO3为前驱体,通过固相合成法制备得到多层g-C3N4负载垂直排列的MoS2(图 4),二维半导体因其结构特性与g-C3N4异质耦合,增加了光催化剂的结构的可调控性,为g-C3N4基异质耦合光催化剂在污染物处理方面提供了更广泛的研究思路。
铋系半导体作为高效可见光驱动光催化剂,在环境净化和修复方面特别是在有机污染物降解方面具有突出的应用前景[46]。氧卤化铋BiOX(X=Cl, Br, I)是一种具有间接带隙跃迁与层状结构的可见光半导体催化剂,具有较高催化稳定性和结构可调控性。[Bi2O2]2+层与双X-层交错形成[-X-Bi-O-O-O-Bi-X-]堆叠层状结构,层与层之间形成的内置静电场能有效分离光生电子-空穴;此外,BiOX的间接带隙跃迁使得光激发电荷需经过一定空间距离才能到达价带,从而降低了光生电子-空穴的复合效率[47]。Wu等[48]通过水热法合成类花状g-C3N4/BiOBr异质耦合光催化剂,如图 5(a), (b)所示,纯BiOBr是由常规纳米薄片自组装而成的规则花状形态,g-C3N4与BiOBr的复合交织,有利于两组分充分接触巩固异质结的形成。g-C3N4/BiOBr在可见光照射下对双酚A的降解效率达到了96.6%,高于纯g-C3N4与BiOBr。目前,2D/2D超薄纳米片异质结已成功构建并在污染物降解方面展现出巨大潜力,但2D/2D纳米片往往需要通过两步水热法或表面活性剂辅助制备,无法达到经济环保的要求。因此2D/2D纳米片的制备仍然是一个具有挑战性的课题。Zhang等[49]在无表面活性剂辅助的前提下,利用一步水热法合成2D/2D BiOCl/g-C3N4纳米片异质结,如图 5(c), (d)所示,不规则椭圆状的BiOCl在g-C3N4表面堆积并具有较完整的接触界面。与纯BiOCl和g-C3N4相比,BiOCl/g-C3N4复合光催化剂的光吸收范围明显拓宽,并对Rh-B的降解效率分别提高3.9, 12.87倍。
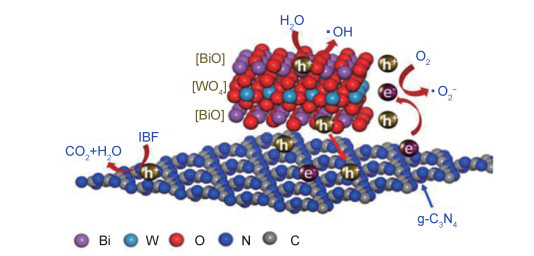
以BiVO4, Bi2WO6为代表的铋系双金属氧化物是一类稳定性能好、禁带宽度小的可见光半导体催化材料,Wang等[50]利用静电纺丝技术制备g-C3N4/BiVO4纳米复合膜并对其在交变视光照射下的光电流响应能力进行评价。结果表明,g-C3N4, BiVO4, g-C3N4/BiVO4膜在大电位范围的光电流是在可见光辐照下产生的,与g-C3N4膜和BiVO4膜相比,g-C3N4/BiVO4膜的整个电位范围内的光电流较高。Wang等[51]利用超薄g-C3N4纳米片和单层Bi2WO6水热反应制备一种新型原子尺度异质结催化剂(UTCB), 该异质结是单层Bi2WO6在g-C3N4纳米片上的组装体,具有较高的光生载流子分离效率。基于光催化降解布洛芬(IBF)的实验结果与DFT理论计算得出其催化机理(图 6),上表面[BiO]+层产生的空穴直接与IBF反应将H2O氧化成·OH,下表面产生的空穴转移至表面与IBF反应生成CO2和H2O,光生电子在中间层[WO4]2+形成,并与由g-C3N4转移至Bi2WO6中间层的光生电子参与还原反应,该催化剂对IBF的降解效率达到96.1%,高于纯g-C3N4纳米片与单层Bi2WO6,由此可见,二者的相互作用显著提高了材料的光催化活性。
以氧化石墨烯(GO)、碳量子点(CQDs)、碳纳米管(CNT)为代表的碳材料因其具有良好的导电性能和较高的电子储存容量,在光催化领域得到广泛关注。研究者通过各种方法制备出不同类型的碳材料/g-C3N4型异质结催化剂,并对其光催化性能进行研究。Hong等[52]利用低温水热法合成CQDs/g-C3N4异质结催化剂,瞬态光电流响应测试显示0.5%(质量分数,下同) CQDs/g-C3N4与纯g-C3N4相比具有较高的光电流强度,说明经与CQDs异质耦合之后催化剂的光生电子-空穴的复合问题得到改善,光催化活性显著提高。Yu等[53]将g-C3N4在酸液中超声处理得到质子化g-C3N4纳米薄片,并利用溶剂法来构建超薄2D/2D rGO/g-C3N4纳米复合材料,两种材料之间具有较强的界面相互作用和丰富的耦合界面促进了光生载流子的分离效率;与g-C3N4纳米薄片相比,rGO/g-C3N4对甲基橙的降解速率提高2.19倍。
尽管石墨烯等材料被证实在提高光催化性能方面效果显著[54],但是制备过程繁琐、产率低、价格昂贵等缺点限制其进一步应用。生物质衍生生物炭作为一种可再生利用资源,因其具有较大的比表面积和丰富的吸附位点,被广泛用于污染物的吸附。另外,生物质热解形成的非晶态生物炭具有较高的电子储存容量和转移效率,能够促进光生载流子的分离。Pi等[55]将栗叶生物炭与三聚氰胺前驱体混合煅烧得到生物炭/g-C3N4复合材料,能够通过吸附和光催化同时来去除污染物,该复合材料对阳离子染料亚甲基蓝(MB)的去除具有较好效果。Li等[56]采用三聚氰胺与纤维素前驱体一步共热法,制备了由生物炭骨架和花状g-C3N4组成的新型光催化剂(C/CN),C/CN-8对甲醛的降解效率达到84.63%,较纯g-C3N4提高近3倍。电化学阻抗(EIS)结果表明C/CN-x的弧半径小于纯g-C3N4,说明生物炭骨架的引入加速了光生载流子的转移速度,在g-C3N4光催化性能改善中起着关键作用。总体来看,碳材料相对于其他金属半导体对g-C3N4光催化性能的提高有限,其研究方向集中在利用碳材料作为电子受体或载体,来构建三元异质耦合光催化剂,这也是g-C3N4基光催化剂的发展趋势之一。
3 复杂三元异质耦合光催化剂三元异质耦合光催化剂(多组分异质结)是在二元异质耦合催化基础上的进一步结构优化,通常是在二元异质耦合的基础上进行的组合,主要为碳/g-C3N4/无机半导体、贵金属/g-C3N4/无机半导体等形式[19]。与二元异质光催化剂相比,三元异质催化剂能够表现出更强的光吸收能力、更高效的光生载流子分离转移效率;催化剂的正协同作用和不同组分界面间的异质耦合使得g-C3N4基三元异质结具有更好的光催化活性[57],是未来g-C3N4基光催化剂研究的热点之一。目前,基于以上二元异质耦合光催化剂衍生出以碳/二元异质结、贵金属/二元异质结等耦合形式为主的三元异质耦合光催化剂得到广泛的研究。Jiang等[58]利用水热法制备全固态RGO/g-C3N4/BiVO4 Z型三元异质光催化剂。该异质结在可见光照射下对盐酸四环素的降解能力显著增强,分别是二元RGO/g-C3N4, g-C3N4/BiVO4, RGO/BiVO4的1.13, 1.16和1.41倍。光催化性能的提高归因于Z型异质结电荷载体分离形式以及电子介质之间的协同作用,其中RGO作为异质结加速电荷分离的促进剂,从而进一步提高了光催化活性。Lu等[59]合成了g-C3N4/Ag/MoS2三元异质耦合光催化剂,g-C3N4/Ag/MoS2对Rh-B的降解效率是Ag/MoS2和g-C3N4/MoS2的9.43,3.56倍,其中Ag作为h+(g-C3N4)与e-(MoS2)的复合“桥梁”,有效促进该材料的电荷分离。另外,有研究者对碳/贵金属/g-C3N4异质结形式进行了尝试,Wang等[60]合成了Pt/GO/g-C3N4异质结,Pt与GO的协同作用加速g-C3N4中光生电子的转移,相较于g-C3N4,Pt/GO/g-C3N4异质结的光催化活性显著提高。
除碳/二元异质结、贵金属/二元异质结等耦合形式外,越来越多的双半导体/g-C3N4三元异质结光催化剂得到广泛研究。Mousavi等[61]制备的g-C3N4/Fe3O4/BiOI纳米可见光驱动光催化剂对罗丹明B、亚甲基蓝和甲基橙的降解活性分别为g-C3N4的10,22和21倍;通过UV-Vis DRS光谱计算出的三元异质耦合催化剂禁带宽度在1.97~2.7 eV之间,禁带宽度的减小拓宽了催化剂对可见光的响应范围;此外,Fe3O4的加入使得该催化剂具有可磁性分离的性能。磁性三元异质耦合催化剂在增强可见光催化活性的同时,通过外加磁场能够提高光催化剂的可回收利用性能,在实际应用中具有重要价值。Jiang等[62]通过一步煅烧法合成WO3/g-C3N4/Bi2O3三元异质结催化剂,并结合活性自由基团捕获实验以及电子自旋共振技术(ESR)提出一种新的双Z型异质耦合光催化机理: 与传统的三元Z型异质结催化机理不同,位于WO3和Bi2O3导带上的光生电子同时与g-C3N4价带上的空穴结合,这种光生载流子的迁移方式会导致g-C3N4导带中的电子积累,而WO3和Bi2O3价带中的空穴得以保留,使其具有足够的激子结合能实现对污染物的降解。研究者提出的g-C3N4基三元异质结的催化机理为多元异质耦合光催化体系的构建与发展提供了更广泛的研究思路。从总体来看,三元异质耦合光催化剂在光催化性能的提升以及稳定性方面均展现出较高的优越性能。
4 g-C3N4基异质耦合光催化剂在环境污染物降解中的研究进展除前两节中综述的g-C3N4基光催化剂对MB,Rh-B,甲醛,甲基橙(MO)等有机污染物的氧化降解外,目前利用g-C3N4基光催化剂处理环境污染物的主要研究还集中在重金属还原、抗生素降解以及抗菌等方面[63-64],而多元异质耦合光催化剂的构建能显著改善g-C3N4的光催化性能,提高其对污染物的处理能力。
Wu等[65]利用锥状钯(Pd)纳米离子与g-C3N4中N原子的相互作用成功将Pd锚定在g-C3N4表面,经Pd负载后的二元异质结具有较低的费米能级,从而促进g-C3N4导带上的电子转移至Pd纳米锥,实验以甲酸作为空穴受体在可见光条件下将水中毒性较大的Cr(Ⅵ)还原为低毒性的Cr(Ⅲ),其还原反应方程式如下所示:
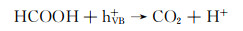
|
(1) |
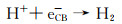
|
(2) |

|
(3) |
结果表明,锥状Pd/g-C3N4具有较高的催化活性,对Cr(Ⅵ)的还原率达到99.9%。Wang等[66]利用纳米零价铁Fe0掺杂MoS2/g-C3N4用于可见光催化还原Cr(Ⅵ),在该催化体系中,Fe0本身具有的还原性能够与MoS2/g-C3N4激发电子产生协同还原作用,加速Cr(Ⅵ)转化为Cr(Ⅲ)。通常情况下,Fe0被消耗并伴随着还原过程而失效,但在该光催化体系下光生电子的还原性将Fe(Ⅲ)/Fe(Ⅱ)还原为Fe0,提高光催化剂的重复利用率。目前利用光催化技术还原重金属Cr(Ⅵ)的研究效果较为显著,但关于g-C3N4还原其他重金属的报道较少,其催化机理仍需进一步探究。
以g-C3N4为代表的无机光催化抗菌剂,通过光催化反应产生一系列具有氧化性的活性物质与细胞结构中的生物大分子反应从而破坏细胞结构来达到灭菌的目的[67-68]。Wu等[69]合成的MnO2/g-C3N4-Ti异质结光催化剂对金黄葡萄球菌和大肠杆菌的灭活率达到99.96%, 99.26%。Xu等[70]利用自组装法将氧化石墨烯量子点(OX-GQDs)修饰氧化g-C3N4(PCNO),实现0D石墨烯量子点在表面以及孔道内的均匀分散,由于OX-GQDs的电子捕获能力使得复合光催化剂的可见光催化消毒性能显著提高,2% X-GQDs/PCNO可使大肠杆菌细胞在可见光照射4 h后失活约99.6%,自由基清除实验和ESR结果表明OX-GQDs/PCNO在光催化反应中产生的活性物质h+, ·O2-和·OH可以有效灭活致病菌。
光催化技术具有能耗较低、反应条件温和、无二次污染等优点,在环境污染修复领域具有广阔的应用前景。目前利用g-C3N4基异质结光催化技术处理污染物的研究仍然处于初级阶段,且主要研究集中在光催化剂的制备以及催化原理的探索中;反应温度、催化时间、催化剂用量、反应体系pH等相关的反应参数对光催化剂在实际应用过程的影响仍需要进一步探究。
5 结束语近年来,研究者对g-C3N4进行了大量的结构、性能调控研究来改善其光催化性能。目前为止,基于g-C3N4光催化剂的研究已经取得了相当大的进展,在环境修复领域具有潜在的应用前景;但在合理制备各种高效g-C3N4基光催化剂以及深入了解g-C3N4异质结光催化的机理方面仍存在诸多挑战。
关于g-C3N4光催化剂的研究,致力于解决两个核心问题:(1)拓宽石墨相氮化碳的光吸收范围,目前的研究主要集中在g-C3N4基异质耦合光催化剂在紫外光-可见光(占太阳光的43%)下的响应,而近红外光(占太阳光的53%)-可见光响应催化剂的设计在理论上具有更广的可见光吸收范围,能在更大程度上增加光激发载流子的数量,是一个值得深入研究的课题。(2)抑制光生载流子的复合效率,构建异质耦合光催化剂是目前最广泛的研究策略之一,其关键在于各组分之间能带匹配以及材料界面接触效果,异质结的成功构建能够提高光生载流子的分离转移效率,因此,构建能带匹配度高的异质耦合光催化剂成为光催化领域的热点。
此外,复杂异质结构中各组分之间的协同效应也应更具体地进行解释;同时需要对电荷转移途径有更深入的理解,并通过相关的表征方法更好地进行验证。在环境污染物降解领域,设计合成具有优异性能的可见光驱动g-C3N4光催化体系仍是一个亟待解决的问题。
| [1] |
JIANG N, DU Y, JI P, et al. Enhanced photocatalytic activity of novel TiO2/Ag/MoS2/Ag nanocomposites for water-treatment[J]. Ceramics International, 2020, 46: 4889-4896. DOI:10.1016/j.ceramint.2019.10.225 |
| [2] |
SALAZAR H, MARTINS P M, SANTOS B, et al. Photocatalytic and antimicrobial multifunctional nanocomposite membranes for emerging pollutants water treatment applications[J]. Chemosphere, 2020, 250: 126299. DOI:10.1016/j.chemosphere.2020.126299 |
| [3] |
RONO N, KIBET J K, MARTINCIGH B S, et al. A review of the current status of graphitic carbon nitride[J]. Critical Reviews in Solid State and Materials Sciences, 2020, 1-29. |
| [4] |
GHOSH U, PAL A. Graphitic carbon nitride based Z scheme photocatalysts: design considerations, synthesis, characterization and applications[J]. Journal of Industrial and Engineering Chemistry, 2019, 79: 383-408. DOI:10.1016/j.jiec.2019.07.014 |
| [5] |
WEN J, XIE J, CHEN X, et al. A review on g-C3N4-based photocatalysts[J]. Applied Surface Science, 2017, 391: 72-123. DOI:10.1016/j.apsusc.2016.07.030 |
| [6] |
ZHANG L, LIU D, GUAN J, et al. Metal-free g-C3N4 photocatalyst by sulfuric acid activation for selective aerobic oxidation of benzyl alcohol under visible light[J]. Materials Research Bulletin, 2014, 59: 84-92. DOI:10.1016/j.materresbull.2014.06.021 |
| [7] |
XIA W, HUANG F, WEI J, et al. Ultrasound exfoliation of g-C3N4 and hydrothermalsynthesis of rGO/g-C3N4 hybrid nanocomposites with improved visible photocatalytic activities[J]. Nanoscience and Nanotechnology Letters, 2017, 9: 1665-1672. DOI:10.1166/nnl.2017.2537 |
| [8] |
XIA P, LIU M, CHENG B, et al. Dopamine modified g-C3N4 and its enhanced visible-light photocatalytic H2-production activity[J]. ACS Sustainable Chemistry & Engineering, 2018, 6: 8945-8953. |
| [9] |
LV H, HUANG Y, KOODALI R T, et al. Synthesis of sulfur doped 2D graphitic carbon nitride nanosheets for efficient photocatalytic degradation of phenol and hydrogen evolution[J]. ACS Appl Mater Interfaces, 2020, 12(11): 12657-12667. |
| [10] |
MISHRA A, MEHTA A, BASU S, et al. Graphitic carbon nitride (g-C3N4)-based metal-free photocatalysts for water splitting: a review[J]. Carbon, 2019, 149: 693-721. DOI:10.1016/j.carbon.2019.04.104 |
| [11] |
RAIZADA P, KUMAR A, SINGH P. Graphitic carbon nitride based new advanced materials for photocatalytic applications[J]. Current Analytical Chemistry, 2020, 16: 1-100. DOI:10.2174/157341101601191216151155 |
| [12] |
LIU C, TANG YB, HUO PW, et al. Novel AgCl/CNTs/g-C3N4 nanocomposite with high photocatalytic and antibacterial activity[J]. Materials Letters, 2019, 257: 126708. DOI:10.1016/j.matlet.2019.126708 |
| [13] |
JIN C, LI W, CHEN Y, et al. Efficient photocatalytic degradation and adsorption of tetracycline over type-Ⅱ heterojunctions consisting of ZnO nanorods and K-doped exfoliated g-C3N4 nanosheets[J]. Industrial & Engineering Chemistry Research, 2020, 59: 2860-2873. |
| [14] |
WANG G, ZHOU F, YUAN B, et al. Strain-tunable visible-light-responsive photocatalytic properties of two-dimensional CdS/g-C3N4: a hybrid density functional study[J]. Nanomaterials (Basel), 2019, 9(2): 244-254. DOI:10.3390/nano9020244 |
| [15] |
TIAN N, ZHANG Y, LI X, et al. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution[J]. Nano Energy, 2017, 38: 72-81. DOI:10.1016/j.nanoen.2017.05.038 |
| [16] |
FU J, YU J, JIANG C, et al. g-C3N4-based heterostructured photocatalysts[J]. Advanced Energy Materials, 2018, 8(3): 1701503. DOI:10.1002/aenm.201701503 |
| [17] |
REN Y, ZENG D, ONG WJ. Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: a review[J]. Chinese Journal of Catalysis, 2019, 40: 289-319. DOI:10.1016/S1872-2067(19)63293-6 |
| [18] |
LOW J, JIANG C, CHENG B, et al. A review of direct Z-scheme photocatalysts[J]. Small Methods, 2017, 1: 1-21. |
| [19] |
ZHANG S, GU P, MA R, et al. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: a critical review[J]. Catalysis Today, 2019, 335: 65-77. DOI:10.1016/j.cattod.2018.09.013 |
| [20] |
QIN J, HUO J, ZHANG P, et al. Improving the photocatalytic hydrogen production of Ag/g-C3N4 nanocomposites by dye-sensitization under visible light irradiation[J]. Nanoscale, 2016, 8: 2249-2459. DOI:10.1039/C5NR06346A |
| [21] |
FU Y, HUANG T, JIA B, et al. Reduction of nitrophenols to aminophenols under concerted catalysis by Au/g-C3N4 contact system[J]. Applied Catalysis B: Environmental, 2017, 202: 430-437. DOI:10.1016/j.apcatb.2016.09.051 |
| [22] |
SHI L, LIANG L, MA J, et al. Remarkably enhanced photocatalytic activity of ordered mesoporous carbon/g-C3N4 composite photocatalysts under visible light[J]. Dalton Trans, 2014, 43: 7236-7244. DOI:10.1039/C4DT00087K |
| [23] |
WANG Y, WANG F, FENG Y, et al. Facile synthesis of carbon quantum dots loaded with mesoporous g-C3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics[J]. Dalton Trans, 2018, 47: 1284-1293. DOI:10.1039/C7DT04360K |
| [24] |
YU J, WANG K, XIAO W, et al. Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4-Pt nanocomposite photocatalysts[J]. Phys Chem Chem Phys, 2014, 16: 11492-11501. DOI:10.1039/c4cp00133h |
| [25] |
LIU G Z C, KANG Y. Unique physicochemical properties of two-dimensional light absorbers faciliating photocatalysis[J]. Chemical Society Reviews, 2018, 47(16): 6410-6444. DOI:10.1039/C8CS00396C |
| [26] |
DAGHRIR R, DROGUI P, ROBERT D. Modified TiO2 for environmental photocatalytic applications: a review[J]. Industrial & Engineering Chemistry Research, 2013, 52: 3581-3599. |
| [27] |
TONG Z, YANG D, XIAO T, et al. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation[J]. Chemical Engineering Journal, 2015, 260: 117-125. DOI:10.1016/j.cej.2014.08.072 |
| [28] |
WANG J, CHEN R, XIANG L, et al. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: a review[J]. Ceramics International, 2018, 44: 7357-7377. DOI:10.1016/j.ceramint.2018.02.013 |
| [29] |
ZHONG Q, LAN H, ZHANG M, et al. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(Ⅵ) and eosin)[J]. Ceramics International, 2020, 46(8): 12192-12199. DOI:10.1016/j.ceramint.2020.01.265 |
| [30] |
KATSUMATA H, TACHI Y, SUZUKI T, et al. Z-scheme photocatalytic hydrogen production over WO3/g-C3N4composite photocatalysts[J]. RSC Adv, 2014, 4: 21405-21409. DOI:10.1039/C4RA02511C |
| [31] |
XIAO T, TANG Z, YANG Y, et al. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics[J]. Applied Catalysis B: Environmental, 2018, 220: 417-428. DOI:10.1016/j.apcatb.2017.08.070 |
| [32] |
DADIGALA R, BANDI R, GANGAPURAM B R, et al. Fabrication of novel 1D/2D V2O5/g-C3N4 composites as Z-scheme photocatalysts for CR degradation and Cr (Ⅵ) reduction under sunlight irradiation[J]. Journal of Environmental Chemical Engineering, 2019, 7(1): 102822. DOI:10.1016/j.jece.2018.102822 |
| [33] |
BHUYAN B, KOIRI D J, DEVI M, et al. A novel MnFe2O4/graphitic carbon nitride (g-C3N4) nanocomposites as efficient magnetically retrievable catalyst in crossed aldol condensation[J]. Materials Letters, 2018, 218: 99-102. DOI:10.1016/j.matlet.2018.01.168 |
| [34] |
PALANIVEL B, AYAPPAN C, JAYARAMAN V, et al. Inverse spinel NiFe2O4 deposited g-C3N4nanosheet for enhanced visible light photocatalytic activity[J]. Materials Science in Semiconductor Processing, 2019, 100: 87-97. DOI:10.1016/j.mssp.2019.04.040 |
| [35] |
ZHOU X, JIN B, CHEN R, et al. Synthesis of porous Fe3O4/g-C3N4 nanospheres as highly efficient and recyclable photocatalysts[J]. Materials Research Bulletin, 2013, 48: 1447-1452. DOI:10.1016/j.materresbull.2012.12.038 |
| [36] |
DUAN Y. Facile preparation of CuO/g-C3N4 with enhanced photocatalytic degradation of salicylic acid[J]. Materials Research Bulletin, 2018, 105: 68-74. DOI:10.1016/j.materresbull.2018.04.038 |
| [37] |
KE Y, GUO H, WANG D, et al. ZrO2/g-C3N4 with enhanced photocatalytic degradation of methylene blue under visible light irradiation[J]. Journal of Materials Research, 2014, 29: 2473-2482. DOI:10.1557/jmr.2014.276 |
| [38] |
XUE S, WU C, PU S, et al. Direct Z-scheme charge transfer in heterostructured MoO3/g-C3N4 photocatalysts and the generation of active radicals in photocatalytic dye degradations[J]. Environ Pollut, 2019, 250: 338-345. DOI:10.1016/j.envpol.2019.04.010 |
| [39] |
HUANG S, XU Y, XIE M, et al. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 478: 71-80. |
| [40] |
LIU K, LI J, YAN X, et al. Synthesis of direct Z-scheme MnWO4/g-C3N4 photocatalyst with enhanced visible light photocatalytic activity[J]. Nano, 2017, 12(10): 1750129. DOI:10.1142/S1793292017501296 |
| [41] |
CHEN L, XU Y, CHEN B. In situ photochemical fabrication of CdS/g-C3N4 nanocomposites with high performance for hydrogen evolution under visible light[J]. Applied Catalysis B: Environmental, 2019, 256: 117848-117856. DOI:10.1016/j.apcatb.2019.117848 |
| [42] |
JIANG F, YAN T, CHEN H, et al. A g-C3N4-CdS composite catalyst with high visible-light-driven catalytic activity and photostability for methylene blue degradation[J]. Applied Surface Science, 2014, 295: 164-172. DOI:10.1016/j.apsusc.2014.01.022 |
| [43] |
LI J, LIU E, MA Y, et al. Synthesis of MoS2/g-C3N4 nanosheets as 2D heterojunction photocatalysts with enhanced visible light activity[J]. Applied Surface Science, 2016, 364: 694-702. DOI:10.1016/j.apsusc.2015.12.236 |
| [44] |
GAO H, LIU Y, WANG L, et al. Synthesis of a reticular porous MoS2/g-C3N4 heterojunction with enhanced visible light efficiency in photocatalytic degradation of RhB[J]. Research on Chemical Intermediates, 2019, 45: 3687-3703. DOI:10.1007/s11164-019-03815-2 |
| [45] |
BIAN H, JI Y, YAN J, et al. In situ synthesis of few-layered g-C3N4 with vertically aligned MoS2 loading for boosting solar-to-hydrogen generation[J]. Small, 2018, 14(3): 1703003. DOI:10.1002/smll.201703003 |
| [46] |
WANG M, ZHANG Y, JIN C, et al. Fabrication of novel ternary heterojunctions of Pd/g-C3N4/Bi2MoO6 hollow microspheres for enhanced visible-light photocatalytic performance toward organic pollutant degradation[J]. Separation and Purification Technology, 2019, 211: 1-9. DOI:10.1016/j.seppur.2018.09.061 |
| [47] |
YAO W, ZHANG J, WANG Y, et al. Hybrid density functional study on the mechanism for the enhanced photocatalytic properties of the ultrathin hybrid layered nanocomposite g-C3N4/BiOCl[J]. Applied Surface Science, 2018, 435: 1351-1360. DOI:10.1016/j.apsusc.2017.11.259 |
| [48] |
WU J, XIE Y, LING Y, et al. Synthesis of flower-like g-C3N4/BiOBr and enhancement of the activity for the degradation of bisphenol under visible light irradiation[J]. Frontiers in Chemistry, 2019, 7: 1-12. DOI:10.3389/fchem.2019.00001 |
| [49] |
ZHANG X, AN D, FENG D, et al. In situ surfactant-free synthesis of ultrathin BiOCl/g-C3N4 nanosheets for enhanced visible-light photodegradation of rhodamine B[J]. Applied Surface Science, 2019, 476: 706-715. DOI:10.1016/j.apsusc.2019.01.147 |
| [50] |
WANG Y S J, LI J, ZHAO X. Electrospinning preparation of nanostructured g-C3N4/BiVO4 composite films with an enhanced photoelectrochemical performance[J]. Langmuir, 2017, 33(19): 4694-4701. DOI:10.1021/acs.langmuir.7b00893 |
| [51] |
WANG J, TANG L, ZENG G, et al. Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation[J]. Applied Catalysis B: Environmental, 2017, 209: 285-294. DOI:10.1016/j.apcatb.2017.03.019 |
| [52] |
HONG Y, MENG Y, ZHANG G, et al. Facile fabrication of stable metal-free CQDs/g-C3N4 heterojunctions with efficiently enhanced visible-light photocatalytic activity[J]. Separation and Purification Technology, 2016, 171: 229-237. DOI:10.1016/j.seppur.2016.07.025 |
| [53] |
YU K, HU X, YAO K, et al. Preparation of an ultrathin 2D/2D rGO/g-C3N4 nanocomposite with enhanced visible-light-driven photocatalytic performance[J]. RSC Advances, 2017, 7: 36793-36799. DOI:10.1039/C7RA06210A |
| [54] |
杨程, 时双强, 郝思嘉, 等. 石墨烯光催化材料及其在环境净化领域的研究进展[J]. 材料工程, 2020, 48(7): 1-13. YANG C, SHI S Q, HAO S J, et al. Research progress in graphene based photocatalytic materials and applications in environmental purification[J]. Journal of Materials Engineering, 2020, 48(7): 1-13. |
| [55] |
PI L, JIANG R, ZHOU W, et al. g-C3N4 Modified biochar as an adsorptive and photocatalytic material for decontamination of aqueous organic pollutants[J]. Applied Surface Science, 2015, 358: 231-239. DOI:10.1016/j.apsusc.2015.08.176 |
| [56] |
LI X, QIAN X, AN X, et al. Preparation of a novel composite comprising biochar skeleton and "chrysanthemum" g-C3N4 for enhanced visible light photocatalytic degradation of formaldehyde[J]. Applied Surface Science, 2019, 487: 1262-1270. DOI:10.1016/j.apsusc.2019.05.195 |
| [57] |
CHENG F, YIN H, XIANG Q. Low-temperature solid-state preparation of ternary CdS/g-C3N4/CuS nanocomposites for enhanced visible-light photocatalytic H2-production activity[J]. Applied Surface Science, 2017, 391: 432-439. DOI:10.1016/j.apsusc.2016.06.169 |
| [58] |
JIANG D, XIAO P, SHAO L, et al. RGO-promoted all-solid-state g-C3N4/BiVO4 Z-scheme heterostructure with enhanced photocatalytic activity toward the degradation of antibiotics[J]. Industrial & Engineering Chemistry Research, 2017, 56: 8823-8832. |
| [59] |
LU D, WANG H, ZHAO X, et al. Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen[J]. ACS Sustainable Chemistry & Engineering, 2017, 5: 1436-1445. |
| [60] |
WANG P, GUAN Z, LI Q, et al. Efficient visible-light-driven photocatalytic hydrogen production from water by using Eosin Y-sensitized novel g-C3N4/Pt/GO composites[J]. Journal of Materials Science, 2017, 53: 774-786. |
| [61] |
MOUSAVI M, HABIBI-YANGJEH A. Magnetically separable ternary g-C3N4/Fe3O4/BiOI nanocomposites: novel visible-light-driven photocatalysts based on graphitic carbon nitride[J]. J Colloid Interface Sci, 2016, 465: 83-92. DOI:10.1016/j.jcis.2015.11.057 |
| [62] |
JIANG L, YUAN X, ZENG G, et al. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant[J]. Applied Catalysis B: Environmental, 2018, 227: 376-385. DOI:10.1016/j.apcatb.2018.01.042 |
| [63] |
PATNAIK S, DAS K K, MOHANTY A, et al. Enhanced photo catalytic reduction of Cr (Ⅵ) over polymer-sensitized g-C3N4/ZnFe2O4 and its synergism with phenol oxidation under visible light irradiation[J]. Catalysis Today, 2018, 315: 52-66. DOI:10.1016/j.cattod.2018.04.008 |
| [64] |
MATHIALAGAN A, MANAVALAN M, VENKATACHALAM K, et al. Fabrication and physicochemical characterization of g-C3N4/ZnO composite with enhanced photocatalytic activity under visible light[J]. Optical Materials, 2020, 100: 109643. DOI:10.1016/j.optmat.2019.109643 |
| [65] |
WU J H, SHAO F Q, LUO X Q, et al. Pd nanocones supported on g-C3N4: an efficient photocatalyst for boosting catalytic reduction of hexavalent chromium under visible-light irradiation[J]. Applied Surface Science, 2019, 471: 935-942. DOI:10.1016/j.apsusc.2018.12.075 |
| [66] |
WANG X, HONG M, ZHANG F, et al. Recyclable nanoscale zero valent iron doped g-C3N4/MoS2 for efficient photocatalysis of RhB and Cr(Ⅵ) driven by visible light[J]. ACS Sustainable Chemistry & Engineering, 2016, 4: 4055-4063. |
| [67] |
FENG T, LIANG J, MA Z, et al. Bactericidal activity and mechanisms of BiOBr-AgBr under both dark and visible light irradiation conditions[J]. Colloids Surf B Biointerfaces, 2018, 167: 275-283. DOI:10.1016/j.colsurfb.2018.04.022 |
| [68] |
ZHAO S W, ZHENG M, SUN H L, et al. Construction of heterostructured g-C3N4/ZnO/cellulose and its antibacterial activity: experimental and theoretical investigations[J]. Dalton Trans, 2019, 49(12): 3723-3734. |
| [69] |
WU B, LI Y, SU K, et al. Corrigendum to "The enhanced photocatalytic properties of MnO2/g-C3N4 heterostructure for rapid sterilization under visible light"[J]. J Hazard Mater, 2020, 384: 121504. DOI:10.1016/j.jhazmat.2019.121504 |
| [70] |
XU J, HUANG J, WANG Z, et al. Enhanced visible-light photocatalytic degradation and disinfection performance of oxidized nanoporous g-C3N4 via decoration with graphene oxide quantum dots[J]. Chinese Journal of Catalysis, 2020, 41(3): 474-484. DOI:10.1016/S1872-2067(19)63501-1 |
 2021, Vol. 49
2021, Vol. 49