文章信息
- 鲍艳, 高璐, 史秀娟, 贾顺田
- BAO Yan, GAO Lu, SHI Xiu-juan, JIA Shun-tian
- 空心柱状CuS的制备及其降解染料性能
- Synthesis of hollow column-like CuS and its dye degradation property
- 材料工程, 2021, 49(2): 136-142
- Journal of Materials Engineering, 2021, 49(2): 136-142.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2020.000084
-
文章历史
- 收稿日期: 2020-01-29
- 修订日期: 2020-07-27
2. 陕西科技大学 轻化工程国家级实验教学示范中心, 西安 710021
2. National Demonstration Center for Experimental Light Chemistry Engineering Education, Shaanxi University of Science and Technology, Xi'an 710021, China
随着纺织技术的飞速发展和生产规模的不断扩大,纺织工业所产生的工业废水也日益增多。据报道,纺织工业的废水排放在我国工业废水中位居第六。然而,纺织工业所产生的废水有80%来自印染[1]。其中,排放的大量偶氮染料不仅难降解、色度高、成分复杂[2],还易于生成致癌性芳香胺。
目前,降解偶氮染料的方法主要有:吸附法[3-4]、膜分离法[5]、光催化法[6-8]、化学氧化法[9]及电化学法[10-12]等。化学氧化法中的芬顿氧化技术由于具有适用范围宽、效率高、操作过程简单等优点,成为工业上最常使用的一类方法。它主要是利用Fe2+催化H2O2,产生具有强氧化能力的羟基自由基(HO·),从而将染料分子直接矿化为CO2,H2O或分解为小分子物质,实现染料的快速高效降解[13]。然而,传统的芬顿氧化技术易产生大量铁污泥,会造成二次污染[14]。因此,改造传统芬顿氧化技术已成为环境工程的热点。现有的改造主要集中在两方面:一是向反应体系中引入电、光、声等条件促进羟基自由基的生成,提高降解效率,即电-芬顿体系[15]、光-芬顿体系[16]、声-芬顿体系[17]及光-电-芬顿体系[18]等;二是发展非均相类芬顿催化剂,制备含铁元素的固体材料或采用其他过渡金属元素(Cu2+,Mn2+)代替Fe2+,减少铁污泥的产生,如BiFeO3[19],CuFeO2[20],Cu2O[21]及Cu/Al2O3[22]等。
纳米CuS是一种应用广泛的半导体材料,具有良好的可见光吸收效应和块体材料无法比拟的光电特性[23],但CuS在光的激发下光生载流子的分离效率较低,因此难以作为光催化剂进行使用。但是,据文献报道纳米CuS在类芬顿催化体系中对有机污染物具有优异的降解特性[24-26],且可避免铁污泥的产生,但现有研究大多集中在采用实心结构的CuS[27-29]。与实心结构相比,空心结构的CuS具有高比表面积、质轻等优点,故备受关注。目前为止,空心球状[30-34]、空心盒状[35]、空心花状[27]及空心方体[36-38]的CuS已成功制备,且在光催化[30]、电池[27, 35, 37]及诊疗[38]等领域有所应用。如Chen等[35]采用沉淀法制备出Cu2O,再以其为模板成功获得空心盒状CuS;将此形貌的CuS作为无锂电池电极,可赋予锂电池优异的循环寿命。Jiang等[30]通过溶剂热法成功制备出粒径约为1.0 μm的空心球状CuS,其与H2O2形成类芬顿催化剂,可显著降解亚甲基蓝溶液。
鉴于此,本工作以硝酸铜、硫代硫酸钠为原料,采用沉淀法制备空心柱状CuS,并以甲基橙染料(MO)为目标污染物,将空心柱状CuS与H2O2形成类芬顿催化体系对其进行降解,研究降解性能。
1 实验材料与方法 1.1 实验试剂及仪器三水硝酸铜(Cu(NO3)2·3H2O)、五水硫代硫酸钠(Na2S2O3·5H2O)及乙二醇(C2H6O2),分析纯,天津市天力化学试剂有限公司;甲基橙(MO),分析纯,天津市河东区红岩试剂厂;无水乙醇(C6H8O7)和过氧化氢(H2O2),分析纯,天津市精细富宇化工有限公司。
D/max-2200型X射线衍射仪(XRD);S-4800型扫描电子显微镜(SEM);FEI Tecnai G2 F20 S-TWIN透射电镜(TEM);VECTOR-22型傅里叶红外光谱测定仪(FT-IR);Cary-5000型紫外-可见分光光度计(UV-vis);BL-GHX-V型光化学反应仪;TG16-WS型台式高速离心机。
1.2 空心柱状CuS的制备及表征将Cu(NO3)2·3H2O和Na2S2O3·5H2O溶于乙二醇和去离子水组成的混合溶剂中并搅拌,待体系颜色变为黄绿色后,70 ℃下持续搅拌2 h。最后,将反应物冷却至室温,离心、醇洗、烘干得到空心柱状CuS。
采用SEM对空心柱状CuS的微观形貌进行观察;采用XRD和FT-IR对空心柱状CuS的物相及组成进行分析。
1.3 空心柱状CuS降解染料的研究空心柱状CuS降解甲基橙(MO)染料的具体操作如下:首先,将20 mg的空心柱状CuS加入含有50 mL甲基橙溶液(浓度为10 mg/L)的石英试管中;然后,将石英试管放置于光化学反应仪中,磁力搅拌下暗反应30 min,使之达到空心柱状CuS与甲基橙的吸附-脱附平衡;随后,加入1 mL质量分数为30%的H2O2溶液并打开光源(以氙灯为光源),每隔20 min从试管中取出6 mL甲基橙溶液,离心(8000 r/min,20 min)后将上清液置于石英比色皿中,通过UV-vis测定其在200~800 nm波长范围内的吸光度,并结合式(1)计算染料的降解率。

|
(1) |
式中:C0为甲基橙溶液的初始吸光度;Ct为不同时间下甲基橙脱色后溶液的吸光度。
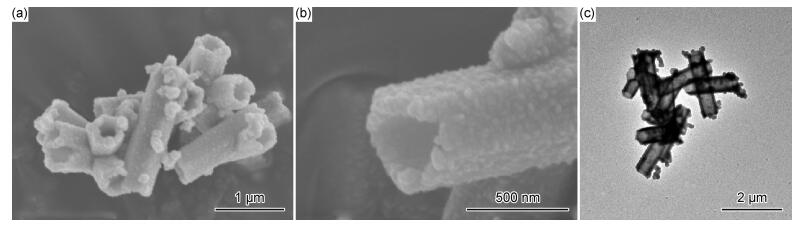
2 结果与分析 2.1 空心柱状CuS的形貌及结构采用SEM和TEM对所制备的空心柱状CuS的形貌进行了表征。图 1(a),(b)是空心柱状CuS的SEM照片,从图 1(a)中可以看出,所制备的硫化铜整体呈六棱柱状且具有显著的空腔结构,表面附着有一些纳米CuS颗粒,粒径较不均一。空心柱状CuS的整体尺寸较大,直径约为400 nm,长度为2.0 μm左右。图 1(b)显示空心柱状CuS的壳层是由不规则的CuS纳米颗粒自组装而成,厚度约为160 nm。此外,图 1(c)给出了空心柱状CuS的TEM照片。可以看出,空心柱状CuS内部比外部亮,成功证实空心结构的形成,且整体尺寸约为2.0 μm,内径约为110 nm,与SEM分析结果一致。
|
图 1 空心柱状CuS的SEM照片(a),(b)和TEM照片(c) Fig. 1 SEM images (a), (b) and TEM image (c) of hollow column-like CuS |
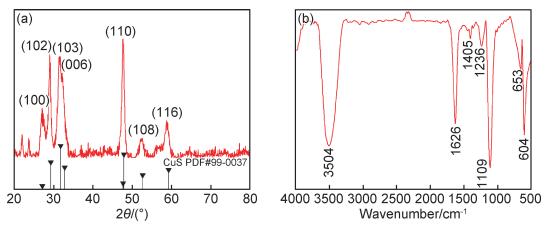
为了进一步分析所制备的空心柱状CuS的结构及化学组成,对其进行了XRD,FT-IR及UV-vis表征。图 2(a)是所制备的空心柱状CuS的XRD谱图。可以看出,2θ为26.9°,29.0°,31.5°,31.7°,47.7°,52.6°和58.8°的衍射峰分别与六角相结构CuS(PDF#99-0037)的(100), (102), (103), (006), (110), (108)和(116)晶面的衍射峰相对应,表明所获得的产物的确为CuS。其中,(110)晶面的衍射峰强度最大,表明所制备的空心柱状CuS是沿着(110)晶面生长的。而FT-IR表征结果如图 2(b)所示。可以清楚地看到,604 cm-1和653 cm-1处出现了Cu—S的伸缩振动吸收峰[39-40],表明硝酸铜及硫代硫酸钠反应生成了CuS。样品在3504 cm-1和1626 cm-1处存在较强的吸收峰,其分别为—OH的伸缩振动和弯曲振动吸收峰,这可能是由于样品中所吸收的水分子引起的[24, 39];1236 cm-1和1405 cm-1处出现—CH和—CH3的吸收峰,1109 cm-1处出现—C—O的伸缩振动吸收峰,这可能是由于CuS在洗涤过程中表面吸附了少量乙醇导致的。
|
图 2 空心柱状CuS的XRD谱图(a)和FT-IR光谱(b) Fig. 2 XRD pattern (a) and FT-IR spectrum (b) of hollow column-like CuS |
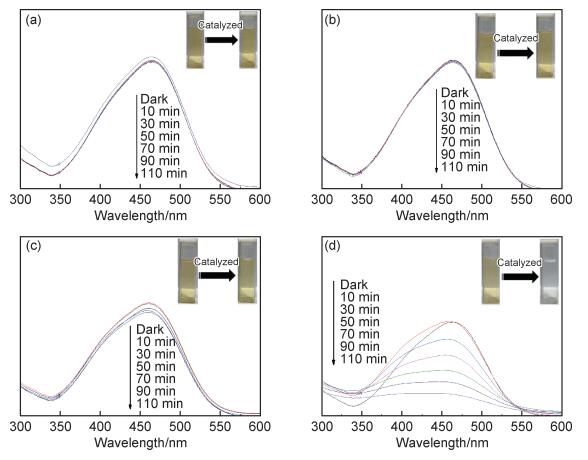
图 3为以氙灯为光源,不同光照时间下甲基橙溶液的UV-vis吸收光谱。从图 3(a),(b)中可以看出,随着光照时间的延长,未加催化剂与仅加H2O2的体系中甲基橙浓度无明显变化,光照前后溶液颜色基本一致。当仅加CuS时,甲基橙溶液的浓度略有降低,但反应结束后甲基橙溶液的颜色仍无明显变化(图 3(c));当H2O2与CuS均加入时,甲基橙的浓度随光照时间的延长明显降低,光照110 min后溶液颜色由黄色变为无色,说明大部分甲基橙被降解(图 3(d))。
|
图 3 光催化降解甲基橙的UV-vis吸收光谱 (a)未加催化剂;(b)仅加H2O2;(c)仅加CuS;(d)CuS与H2O2均加入 Fig. 3 UV-vis absorption spectra of photocatalytic degradation of methyl orange (a)without catalyst; (b)with H2O2; (c)with CuS; (d)with CuS and H2O2 |
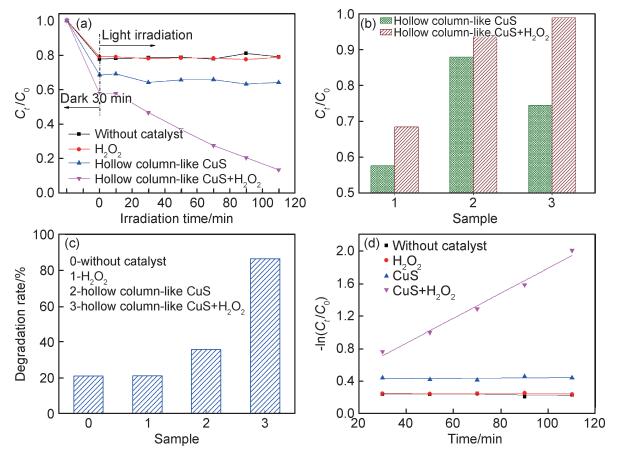
图 4(a)~(d)为根据图 3所获得的不同条件下甲基橙的降解曲线及降解率。从图 4(a)中可以看出,暗反应过程中,含空心柱状CuS的两种催化体系对染料的吸附能力大于未加催化剂体系和仅加H2O2体系的,这是由于所制备的CuS具有显著的空腔结构且其由纳米片组装而成,故比表面积较高,对于甲基橙有良好的吸附作用。但此过程中,含空心柱状CuS的两种催化体系表现出不同的吸附能力。对含空心柱状CuS的两种催化体系在该阶段的反应重复测定了3次,结果如图 4(b)所示。从结果中可以看出,这两种催化体系在每一次的重复实验中对染料的吸附均存在较大差异,这可能是由于空心柱状CuS在不同体系中的分散程度略有不同,在一定时间内对染料的吸附能力不同,故取样后溶液的吸光度不同。
|
图 4 空心柱状CuS光催化降解甲基橙的性能 (a)光催化降解曲线; (b)含空心柱状CuS体系暗反应阶段对染料的吸附图; (c)光催化降解率; (d)动力学拟合曲线 Fig. 4 Photocatalytic degradation of methyl orange by hollow column-like CuS (a)photocatalytic degradation curves; (b)adsorption diagram of dye in dark reaction phase of hollow cylindrical CuS system; (c)photocatalytic degradation rate; (d)kinetic fit curves |
在开启光源后,未加催化剂体系和仅加H2O2体系的吸光度与暗反应结束时的吸光度无明显差别,且随着光照时间的延长吸光度基本没有发生变化,说明这两个体系中基本没有产生羟基自由基,因此无法降解甲基橙。由图 4(c)可知,光反应结束后未加催化剂的体系和仅加H2O2的体系甲基橙的降解率分别为21.1%和21.3%。与这两种体系相比,仅加CuS的体系,随着光照时间的延长,吸光度呈现轻微下降的趋势,甲基橙的降解效果有所提升但依然不佳,降解率也只有35.9%。这是因为CuS作为光催化剂在光的激发下光生载流子的分离效率较低,因此降解活性不高[41]。当同时加入H2O2与CuS时,随着光照时间的延长,吸光度呈现直线下降的趋势,110 min后甲基橙的降解率可高达86.6%。这是因为CuS中的Cu2+与H2O2可组成类Fenton体系,两者反应产生了具有强氧化能力的羟基自由基,进一步氧化甲基橙,从而使其被分解完全。具体的降解原理可以通过反应方程式(2)~(5)进行说明[42]。进一步对图 4(a)进行线性拟合(图 4(d)),可知CuS与H2O2形成的类芬顿催化剂的降解反应速率为0.0154 min-1。此外,将该研究结果与文献中所报道的CuS作为催化剂降解染料的研究结果进行对比[30, 43-47],如表 1所示。可以看出,与文献中所报道的CuS相比,如CuS纳米颗粒[43]、球状CuS[44],CuS纳米片组合体[45]及巢状CuS[46],空心柱状CuS可大大缩短染料的降解时间,表现出优异的染料降解性能。与空心球状CuS[30]相比,空心柱状CuS与其降解能力相当,说明空心结构有助于提升CuS对染料的降解能力。
| Sample | Catalyst dosage | Contamination type | Contamination concentration (mg·L-1) |
Mass ratio of catalyst to contamination | H2O2 dosage/mL | Light source | Degradation ability | Ref |
| Hollow column-like CuS | 20 mg/50 mL | MO | 10 | 40∶1 | 1 | Xenon lamp | 86.6% in 110 min | This work |
| Hollow sphere-like CuS | 30 mg/40 mL | Methylene blue | 20 | 37.5∶1 | 1.3 | Natural light | ≈90% in 90 min | [30] |
| CuS nanoparticles | 30 mg/100 mL | Methylene blue | 15 | 20∶1 | 0 | Visible light | 93% in 180 min | [43] |
| Spheroidal CuS | 30 mg/100 mL | Methylene blue | 25 | 12∶1 | 5 | Visible light | ≈97% in 600 min | [44] |
| CuS nanoplate-based architectures | 20 mg/40 mL | Methylene blue | 20 | 25∶1 | 1.3 | Sun light | ≈80% in 150 min | [45] |
| Nested CuS | 10 mg/200 mL | Rhodamine B | 2.5 | 20∶1 | 0.2 | Xenon lamp | 58.88% in 120 min | [46] |
| CuS nanoparticles CuS nanoplates |
80 mg/100 mL | Methylene blue | 60 | 13.3∶1 | 2 | Xenon lamp | 77.5% in 160 min | [47] |
| 66.3% in 160 min |

|
(2) |
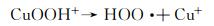
|
(3) |
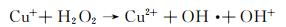
|
(4) |
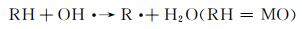
|
(5) |
(1) 采用沉淀法成功制备了六角相结构的空心柱状CuS,其直径在4.0~5.0 μm,长度为10 μm左右。
(2) 将空心柱状CuS用于降解甲基橙溶液,发现空心结构的存在使CuS对甲基橙具有较好的吸附能力。但因其对电子-空穴对的分离效率较低,故仅采用空心柱状CuS为光催化剂时,对甲基橙的降解效果非常弱;但将其与H2O2形成类芬顿试剂时,由于电子-空穴对的复合被抑制,因此表现出优异的降解性能,在110 min时对甲基橙的降解率可高达86.6%,降解反应速率为0.0154 min-1,远优于实心结构CuS对染料的降解能力。
| [1] |
张莉莉, 印染废水脱色处理技术的研究[D]. 苏州: 苏州大学, 2008. ZHANG L L.Research on decolorization treatment of dyeing and printing wastewater[D].Suzhou: Soochow University, 2008. |
| [2] |
蒋浩. 磁性Fe3O4纳米颗粒催化阴极电Fenton降解水中偶氮染料甲基橙研究[D]. 南京: 南京大学, 2017. JIANG H.Cathode electro-Fenton oxidation of azo dye methyl orange catalyzed by magnetic Fe3O4 nanoparticles[D].Nanjing: Nanjing University, 2017. |
| [3] |
葛梦妮, 张建峰, 曹惠杨, 等. 剥离时间对二维Ti3C2吸附染料污染物性能的影响[J]. 材料工程, 2018, 46(7): 144-150. GE M N, ZHANG J F, CAO H Y, et al. Effect of etching time on the adsorption performance of two-dimensional Ti3C2 for organic dyes[J]. Journal of Materials Engineering, 2018, 46(7): 144-150. |
| [4] |
YANG C, CHENG J H, CHEN Y C, et al. Enhanced adsorption performance of MoS2, nanosheet-coated MIL-101 hybrids for the removal of aqueous rhodamine B[J]. Journal of Colloid and Interface Science, 2017, 504(15): 39-47. |
| [5] |
王进, 赵长伟, 吴珍, 等. 氧化石墨烯/聚哌嗪酰胺复合纳滤膜在染料脱除中的应用研究[J]. 膜科学与技术, 2016, 36(6): 86-94. WANG J, ZHAO C W, WU Z, et al. Applied research of graphene oxide polypiperazine-amide composite nanofiltration membrane in removal[J]. Membrane Science and Technology, 2016, 36(6): 86-94. |
| [6] |
DENG H, MAO Z P, XU H, et al. Synthesis of fibrous LaFeO3 perovskite oxide for adsorption of Rhodamine B[J]. Ecotoxicology and Environmental Safety, 2019, 168(30): 35-44. |
| [7] |
刘湘粤, 张宇, 王晨, 等. 亚铁盐制备高结晶度MIL-100(Fe)纳米材料及其光降解有机染料性能[J]. 材料工程, 2018, 46(10): 127-134. LIU X Y, ZHANG Y, WANG C, et al. Highly crystalline MIL-100(Fe) nanoparticles prepared from ferrous salts and applications in photodegradation of organic dyes[J]. Journal of Materials Engineering, 2018, 46(10): 127-134. |
| [8] |
赵晓华, 魏崇, 苏帅, 等. Ag3PO4/ZnO@碳球三元异质结的合成及可见光催化性能[J]. 材料工程, 2019, 47(7): 76-83. ZHAO X H, WEI C, SU S, et al. Synthesis and visible light photocatalytic performance of Ag3PO4/ZnO@carbon sphere ternary heterostructure[J]. Journal of Materials Engineering, 2019, 47(7): 76-83. |
| [9] |
ZHANG S, WANG D, ZHANG S S, et al. Ozonation and carbon-assisted ozonation of methylene blue as model compound: effect of solution pH[J]. Procedia Environmental Sciences, 2013, 18: 493-502. DOI:10.1016/j.proenv.2013.04.066 |
| [10] |
YUE L, WANG K H, GUO J B, et al. Enhanced electrochemical oxidation of dye wastewater with Fe2O3 supported catalyst[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(2): 725-731. DOI:10.1016/j.jiec.2013.06.001 |
| [11] |
范莹莹, 陈阵, 李佳莹, 等. AEO改性二氧化铅电极在降解甲基橙过程中的电化学行为[J]. 材料工程, 2014(7): 10-15. FAN Y Y, CHEN Z, LI J Y, et al. Electrochemical behavior of PbO2 electrode modified by AEO for the degradation of methyl orange[J]. Journal of Materials Engineering, 2014(7): 10-15. |
| [12] |
唐长斌, 卢宇轩, 王飞, 等. 用于水体中有机污染物电催化降解的非贵金属氧化物阳极的研究进展[J]. 材料工程, 2020, 48(6): 62-72. TANG C B, LU Y X, WANG F, et al. Research progress of non-precious metal oxide coated anodes for electrocatalytic degradation of organic pollutants in water[J]. Journal of Materials Engineering, 2020, 48(6): 62-72. |
| [13] |
李春娟. 芬顿法和类芬顿法对水中污染物的去除研究[D]. 哈尔滨: 哈尔滨工业大学, 2009. LI C J.Investigation of removal of contaminants in water by Fenton and Fenton-like oxidatio[D].Harbin: Harbin Institute of Technology, 2009. |
| [14] |
宋昭仪, 胥维昌, 马文静, 等. 偶氮染料废水处理技术及研究进展[J]. 染料与染色, 2018, 55(6): 56-60. SONG Z Y, XU W C, MA W J, et al. Treatment technology and progress of azo-dye waste water[J]. Dyestuffs and Coloration, 2018, 55(6): 56-60. |
| [15] |
ZHAO F, LIU L F, YANG F L, et al. E-Fenton degradation of MB during filtration with Gr/PPy modified membrane cathode[J]. Chemical Engineering Journal, 2013, 230(15): 491-498. |
| [16] |
NGUYEN X S, ZHANG G L, YANG X F. Mesocrystalline Zn-doped Fe3O4 hollow submicrospheres: Formation mechanism and enhanced photo-Fenton catalytic performance[J]. ACS Applied Materials & Interfaces, 2017, 9(10): 8900-8909. |
| [17] |
HSIEH L L, KANG H J, SHYU H Y, et al. Optimal degradation of dye wastewater by ultrasound/Fenton method in the presence of nanoscale iron[J]. Water Science & Technology, 2009, 60(5): 1295-1301. |
| [18] |
周笑绿, 施艺炜, 谢跃, 等. 利用光-电芬顿降解印染废水的研究[J]. 上海电力学院学报, 2016, 32(6): 547-551. ZHOU X L, SHI Y W, XIE Y, et al. Research on printing and dyeing wastewater by light and electric fenton[J]. Journal of Shanghai University of Electric Power, 2016, 32(6): 547-551. |
| [19] |
王晓瑞, 张娜, 陈若潇, 等. 化学共沉淀法制备纯相BiFeO3粉体[J]. 功能材料, 2018, 49(4): 4189-4192. WANG X R, ZHANG N, CHEN R X, et al. Preparation of pure phase BiFeO3 powders by chemical co-precipitation method[J]. Journal of Functional Materials, 2018, 49(4): 4189-4192. |
| [20] |
DAI C, TIAN X K, NIE Y L, et al. Surface facet of CuFeO2 nanocatalyst: a key parameter for H2O2 activation in Fenton-like reaction and organic pollutant degradation[J]. Environmental Science & Technology, 2018, 52(11): 6518-6525. |
| [21] |
王月娇. 微纳尺度氧化亚铜和硫化铜的调控合成及其性能研究[D]. 青岛: 青岛科技大学, 2010. WANG Y J.Controllable synthesis and properties of micro/nanometer Cu2O and C[D].Qingdao: Qingdao University of Science and Technology, 2010. |
| [22] |
谭亚军, 蒋展鹏, 祝万鹏, 等. 用于有机污染物湿式氧化的铜系催化剂活性研究[J]. 化工环保, 2000, 20(3): 6-10. TAN Y J, JIANG Z P, ZHU W P, et al. Study on activity of copper catalysts for the wet oxidation of organic pollutants[J]. Environmental Protection of Chemical Industry, 2000, 20(3): 6-10. |
| [23] |
刘红梅. 硫化铜纳米管的温和条件制备研究[J]. 廊坊师范学院学报(自然科学版), 2013, 13(2): 76-84. LIU H M. On the preparation of copper sulfide nanotubes in mild conditions[J]. Journal of Langfang Normal University(Natural Science Edition), 2013, 13(2): 76-84. |
| [24] |
FAYAZI M, AZIZIAN S. Catalytic degradation of methyl violet without light irradiation using nanostructured CuS[J]. Journal of Molecular Liquids, 2016, 224: 763-767. DOI:10.1016/j.molliq.2016.10.058 |
| [25] |
潘欣子. 自组装构筑硫化铜中空材料及其催化性质的研究[D]. 哈尔滨: 哈尔滨工业大学, 2018. PAN X J.Research on self-assembly technique building hollow copper sulfide and their catalytic properties[D].Harbin: Harbin Institute of Technology, 2018. |
| [26] |
HU X S, SHEN Y, XI L H, et al. Preparation of flower-like CuS by solvothermal method and its photodegradation and UV protection[J]. Journal of Alloys and Compounds, 2016, 674(25): 289-294. |
| [27] |
LIU Y X, ZHOU Z X, ZHANG S P, et al. Controllable synthesis of CuS hollow microflowers hierarchical structures for asymmetric supercapacitors[J]. Applied Surface Science, 2018, 442(1): 711-719. |
| [28] |
QIN Y, KONG X G, LEI D Q, et al. A facial grinding method for the synthesis of high-purity CuS nanosheets[J]. Industrial & Engineering Chemistry Research, 2018, 57(8): 2759-2764. |
| [29] |
宣玉凤. 纳米硫化铜的制备及其性质研究[D]. 天津: 天津大学, 2015. XUAN Y F.CuS nanoparticles: preparation and property studies[D].Tianjin: Tianjin University, 2015. |
| [30] |
JIANG D H, HU W B, WANG H R, et al. Synthesis, formation mechanism and photocatalytic property of nanoplate-based copper sulfide hierarchical hollow spheres[J]. Chemical Engineering Journal, 2012, 189: 443-450. |
| [31] |
BAI L, YE X J, SONG C C, et al. The surfactant-free synthesis of hollow CuS nanospheres via clean Cu2O templates and their catalytic oxidation of dye molecules with H2O2[J]. RSC advances, 2016, 6(87): 83885-83889. DOI:10.1039/C6RA19447H |
| [32] |
DENG C H, GE X Q, HU H M, et al. Template-free and green sonochemical synthesis of hierarchically structured CuS hollow microspheres displaying excellent Fenton-like catalytic activities[J]. Cryst Eng Comm, 2014, 16(13): 2738-2745. DOI:10.1039/C3CE42376J |
| [33] |
HE Y J, YU X J, ZHAIO X L. Synthesis of hollow CuS nanostructured microspheres with novel surface morphologies[J]. Materials Letters, 2007, 61(14/15): 3014-3016. |
| [34] |
MENG X Y, TIAN G H, CHEN Y J, et al. Hierarchical CuS hollow nanospheres and their structure-enhanced visible light photocatalytic properties[J]. CrystEngComm, 2013, 15(25): 5144-5149. DOI:10.1039/c3ce40195b |
| [35] |
CHEN Y W, LI J M, LEI Z W, et al. Hollow CuS nanoboxes as Li-free cathode for high-rate and long-life lithium metal batteries[J]. Advanced Energy Materials, 2020, 10(7): 1903401. DOI:10.1002/aenm.201903401 |
| [36] |
CAI L Y, SUN Y G, LI W Y, et al. CuS hierarchical hollow microcubes with improved visible-light photocatalytic performance[J]. RSC Advances, 2015, 5(119): 98136-98143. DOI:10.1039/C5RA18563G |
| [37] |
SHEN J S, ZHANG Y J, CHEN D, et al. A hollow CuS nanocube cathode for rechargeable Mg batteries: effect of the structure on the performance[J]. Journal of Materials Chemistry A, 2019, 7(37): 21410-21420. DOI:10.1039/C9TA07470H |
| [38] |
ZHU J L, PENG X, NIE W, et al. Hollow copper sulfide nanocubes as multifunctional nanozymes for colorimetric detection of dopamine and electrochemical detection of glucose[J]. Biosensors and Bioelectronics, 2019, 141: 111450. DOI:10.1016/j.bios.2019.111450 |
| [39] |
裴立宅, 王季芬, 陶新秀, 等. 硫化铜晶体的合成及其光学特性[J]. 铜业工程, 2011(2): 24-26. PEI L Z, WANG J F, TAO X X, et al. Synthesis and optical properties of CuS crystals[J]. Copper Engineering, 2011(2): 24-26. |
| [40] |
SARANYA M, SANTHOSH C, RAMACHANDRAN R, et al. Hydrothermal growth of CuS nanostructures and its photocatalytic properties[J]. Powder Technology, 2014, 252: 25-32. DOI:10.1016/j.powtec.2013.10.031 |
| [41] |
马小悦. 金属有机骨架/无机半导体复合材料的制备和性能研究[D]. 南京: 东南大学, 2018. MA X Y.Preparation and properties of metal organic frameworks/inorganic semiconductor composites[D].Nanjing: Southeast University, 2018. |
| [42] |
严登标. 双反应中心类芬顿催化剂CN-Cu(Ⅱ)-CuAlO2制备及对双酚A的降解研究[D]. 天津: 天津工业大学, 2019. YAN D B.Preparation of CN-Cu(Ⅱ)-CuAlO2 Fenton-like catalyst with two reaction centers and study on degradation of bisphenol A[D].Tianjin: Tiangong University, 2019. |
| [43] |
PAL M, MATHEWS N R, SANCHEZ-MORA E, et al. Synthesis of CuS nanoparticles by a wet chemical route and their photocatalytic activity[J]. Journal of Nanoparticle Research, 2015, 17(7): 301. DOI:10.1007/s11051-015-3103-5 |
| [44] |
杨书辉, 祁诗月, 苑大凯, 等. 胞外多聚物调控合成纳米硫化铜及光催化性能[J]. 环境卫生工程, 2019, 27(1): 74-79. YANG S H, QI S Y, YUAN D K, et al. Biosynthesis of CuS nanoparticles by EPS as a mediator and photocatalytic activity[J]. Environmental Sanitation Engineering, 2019, 27(1): 74-79. |
| [45] |
LI F, BI W, KONG T, et al. Optical, photocatalytic properties of novel CuS nanoplate-based architectures synthesised by a solvothermal route[J]. Crystal Research and Technology, 2009, 44(7): 729-735. DOI:10.1002/crat.200800618 |
| [46] |
ZHANG Y Q, ZHANG B P, ZHU L F. Monodisperse CuS nanodisks: low-temperature solvothermal synthesis and enhanced photocatalytic activity[J]. RSC Advances, 2014, 4(103): 59185-59193. DOI:10.1039/C4RA06274D |
| [47] |
王杰. 不同形貌CuS微/纳米结构的制备及其可见光催化性能[D]. 郑州: 郑州大学, 2013. WANG J.Preparation of CuS micro/nano structured materials with different morphologies and its photocatalytic performance[D].Zhengzhou: Zhengzhou University, 2013. |
 2021, Vol. 49
2021, Vol. 49


