文章信息
- 刘梦如, 叶诚曦, 彭黎波, 翁景峥
- LIU Meng-ru, YE Cheng-xi, PENG Li-bo, WENG Jing-zheng
- PAA类黏结剂在锂电池中电化学性能研究进展
- Research progress in electrochemical properties of lithium batteries with PAA binders
- 材料工程, 2021, 49(2): 21-31
- Journal of Materials Engineering, 2021, 49(2): 21-31.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2020.000532
-
文章历史
- 收稿日期: 2020-06-12
- 修订日期: 2020-09-10
2. 福建师范大学 福建高分子重点实验室, 福州 350007
2. Fujian Key Laboratory of Polymer Science, Fujian Normal University, Fuzhou 350007, China
不断增长的能源需求与日益枯竭的矿物燃料资源之间的矛盾,使得具有较高能量密度、循环寿命及更低成本的锂电池成为人们使用最多的能量储存设备之一[1-4]。黏结剂作为锂电池中的重要组成部分,将正负极活性材料、导电剂及集流体连接在一起,保证电极的形态和结构的完整性[5],并为电极中的电子传递提供通道[6]。由于聚合物黏结剂是一种无活性的成分,它只是简单地结合组成粒子来维持电极的形状,这使得研究人员曾认为黏结剂对电池性能的改善不如活性材料[7]。研究认为,黏结剂虽然占比较少[8-9],但对正负极活性材料在充放电过程中的体积变化及结构维持起到非常关键的作用。它可以有效地防止活性材料松动和脱落[10],不仅可以提高极片的循环稳定性[11],还能在一定程度上减小阻抗,提高锂离子扩散速率,并能间接地提高比容量,减少容量损失。
锂电池用黏结剂应具有电化学稳定性好、黏度适中、力学性能好及电解液溶胀率小等特点,并能够促进导电剂和活性物质在浆料中均匀分散,并具有较强的黏结能力,将活性物质等很好地黏结在集流体上,且能够与活性材料一起变形而不会失效[12-13]。此外,还应保证活性材料颗粒表面/电解质界面处的电子和离子电导率[14]。合适的黏结剂可以优化具有较低锂离子扩散速率和电导率的LiFePO4[15]电极性能,改善LiCoMnO4[16],NCM [Li(Ni, CO, Mn)O2] [17-19]等正极极片的功率、循环性能及安全性。对于拥有4200 mAh·g-1高理论比容量的硅负极[12, 20],能在充放电过程中抑制硅体积变化的黏结剂,对提高其电池循环稳定性至关重要。
目前PVDF、PAA、CMC及海藻酸钠等都是常用的锂电池黏结剂。PVDF因化学稳定性好、易于分散、电化学性能好而被广泛用于锂电池中,但其电解液中溶胀率高[13]、力学性能和黏附力较差[14],不利于电池的长期使用, 且溶剂NMP有毒,对环境不友好[21]。CMC中含有大量羟基和羧甲基基团,可通过化学键与硅相连,结合力较强,并能在硅表面形成类似SEI膜的包覆,抑制电解液的分解,断裂伸长率小(5%~8%),不易发生形变[22],且材料来源广,成本低,可降解,对环境友好[23]。但CMC的黏性不高、脆性大、充放电时极片易产生裂纹,且受电极材料配比、pH值等条件的影响较大[13]。丁苯胶乳(SBR)弹性好、耐热性高、约束力强,但其变形能力高,多与CMC混合使用,可获得具有模量小、伸长率高、黏结及分散性能好、电解液吸收率低的CMC-SBR黏结剂体系。海藻酸钠的结构与CMC相似,但其羧基官能团分布更为均匀,且溶胀率低,保形性好,可形成稳定的SEI膜,极性大,黏附力强,可有效防止硅颗粒的团聚和脱落。但其脆性大,材料的稳定性差,不利于电池长期使用[22-23]。
聚丙烯酸(PAA)在2006年开始用作锂电池黏结剂[24],其分子结构简单、易合成,能溶于水、乙醇[25]及NMP等溶剂,可作为锡[26]、硅[27-29]、石墨负极[30-31]和LiFePO4[32-33]、LiMn2O4[34]等正极的黏结剂。其在碳酸盐中具有较低的溶胀性和较高的弹性模量,并因其羧基含量高,可以与活性材料表面的羟基形成强氢键作用[13, 22],故而其黏附力及力学性能较传统黏结剂PVDF更好[25, 35-36],且其可以在活性材料表面形成一层或多或少的涂层,起到人工SEI作用[37],有利于提高电池电性能,是锂电池正负极常用的聚合物黏结剂之一。将交联的PAA黏结剂用于硅负极,增强了Si颗粒与导电材料及集流体之间的黏结力,提高了极片的循环稳定性,其电极在4 A·g-1的电流密度下循环300次后,仍能保持82%的容量[38]。但其链刚性较大,受力后易发生永久变形,而导致活性物质团聚,且亲水力较强[13, 22, 39]。为了尽可能有效地提高电池的电化学性能,研究者们用各种手段对PAA进行了物理化学处理,以更好地用于锂电池电极浆料。本文总结了近几年PAA黏结剂的最新研究进展,包括PAA的结构特性、改性及应用方式和其制备的锂电池的电性能改善情况。
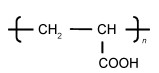
1 PAA黏结剂的结构特性PAA是具有大量羧基的线性长链结构[40-41],其结构式如图 1,用作锂电池黏结剂时的重均分子量Mw通常约450000。羧基使得PAA自身的分子链间形成氢键,产生一定程度的交联,故而黏结性能较好[42],且可以和活性物质颗粒或导电剂颗粒表面的羟基形成酯键及氢键结构[32],增强黏附力,有利于电极的稳定性。
|
图 1 PAA的结构式 Fig. 1 The structural formula of PAA |
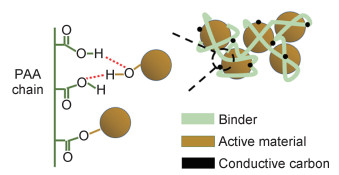
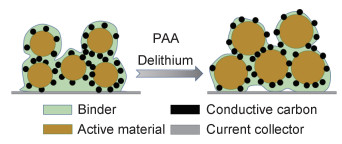
PAA黏结剂为无定形态物质,呈一种不规则的“脑髓状”结构,图 2是PAA黏结剂与活性材料间的作用机理图。即使在干燥过程中也能非常均匀地覆盖在活性物质和导电材料表面,可以很好地阻碍电解液对极片的侵蚀[43]。且因PAA的锂传递特性,使得锂离子可以在邻近的羧基位置[44]间跳跃,故而在硅颗粒表面形成或多或少的薄涂层,发挥人工SEI的作用[45],在一定程度上降低阻抗,提高锂离子扩散速率。图 3为PAA黏结剂解决活性材料体积变化的模型图。
|
图 2 PAA黏结剂与活性材料间的作用机理图 Fig. 2 Mechanism diagram between PAA binder and active material |
|
图 3 PAA黏结剂解决活性材料体积变化的模型图 Fig. 3 Model diagram of PAA binder solving volume change of active material |
PAA的体积热膨胀系数较小,热扩散系数大,其电池在大功率充放电、滥用及高温下使用时,较PVDF基电池更安全,因体积膨胀发生的安全事故的可能性更小,可以避免因温度集中而引发的火灾[43]。此外对电池的使用来说,黏结剂对电解液的吸液程度也非常重要。在电解液中,具有化学及电化学稳定性有利于提高电池的使用寿命及性能;适度的亲和力有利于锂离子在电极内的迁移[46],从而获得更高的锂离子传输速率。而PAA能够较PVDF更有效地抑制活性材料在电解液中的膨胀[13],具有较好的电解液溶胀率及稳定性,可以很好地用作锂电池黏结剂。
但大量羧基的存在,使得PAA极易吸水,且羧基间产生的氢键过多,会阻碍分子链的自由旋转,使得聚合物柔性过差,不利于承受活性物质体积膨胀产生的应力,影响电池性能的提升,且PAA的线性长链结构,使得分子链之间易于滑动,在受力后易发生永久变形而导致活性物质团聚,降低极片的比容量[39]。为了解决PAA黏结剂对性能提升的限制,设计新型改性PAA聚合物黏结剂的方案对锂电池使用性能的提高势在必行。
研究改变PAA链的刚性程度,可引入合适的弹性链结构或通过取代、自由基聚合等方法调整羧基的分布情况,或引入适当的官能团,以减少PAA链间氢键数量。对于PAA的线性长链,需要其自身或与其他聚合物进行适度的交联,增强黏结剂在充放电过程中对活性材料体积变化的承受能力,控制其物理化学交联点的数量,使改性PAA黏结剂具有一定的自愈性以提高电极的恢复能力。对此,可通过接枝[28, 47-48]、增强机械交锁[44, 49]、改善界面结合[50]、引入弹性[51-52]、自愈性[53]和电/离子导电性[54-55]、拓扑交联[56]等[57]方式对PAA进行改性,来更有效地提高锂电池的使用性能。
2 改性PAA的制备及应用方式科学家们首先对纯PAA黏结剂的应用方式进行研究。纯PAA作为黏结剂,可直接运用于浆料中。将PAA与经由TA表面处理的SiSMPs共同加入浆料中,搅拌均匀,得到的电极具有较大的剥离力,其中质量分数为2% TA处理的SiSMPs所在电极的剥离力最大,约为15 N,这对提高电池循环性能具有较大的影响[58]。也有人将PAA直接用于MnO/PD负极浆料[59]及硫化聚丙烯腈(SPAN)正极浆料[60]中,测试在不同活性材料电极中,PAA黏结剂对其电化学性能的影响; 或在浆料中添加其他物质,与PAA共同作用,以更好地提高电池的电化学性能。有研究将PAA与PVA按2∶1的比例(质量比,下同)直接加入LiFePO4/C正极浆料中混合,得到的黏结剂PAA-PVA(2∶1)具有最佳的黏结性能,对电解液的溶胀度也适中,具有较好的电化学性能和循环稳定性[33]。将PAA和GL直接作用于Si@SiO2负极浆料,由于黏结剂PAA-GL具有大量羟基和羧基,与Si@SiO2有着丰富的接触位点,且黏结剂上游离的羧基与Si@SiO2的羟基之间的相互作用,而使获得的电极具有6.44 N的平均剥离力,有利于电极在长期脱嵌锂过程中的稳定性[61]。
2.1 与多种聚合物共混由于聚合物PAA自身结构的限制,于浆料中简单的混合,并不能有效提高电池的使用性能,在加入浆料前,更多地采用多种聚合物与PAA进行物理预混合,改善黏结剂的组成以弥补PAA的结构缺陷。
预先将PAA与PAA/PMA溶解于N,N-二甲基甲酰胺(DMF)中,得到PAA-PAA/PMA黏结剂用于Si/C负极,大大提高了电极的恢复率[62]。PAA和PAI预混合后,用作Si/C负极黏结剂,具有适当的模量,有利于电极的稳定性[63]。而PAA与CMC按1∶1溶液混合后,得到PAA-CMC黏结剂制备的Co3O4电极,因PAA与CMC之间的耦合协同效应而具有较高的容量和稳定性[64]。还有研究采用简单共混制备出PAANa[65],PAA-Rosin[66],ACC-PAA[57],PAA-PANI[67]及WPU-PAA-GN[68]等改性PAA黏结剂,用于不同的正负极中。
PAA进行简单的改性处理,如GA改性PAA[27],或采用可逆加成碎片链转移(RAFT)聚合的方法,将TEGDA与PAA进行聚合,得到不同支化程度的黏结剂PAA-TEGDA,来检测PAA支化程度对电池电化学性能的影响[69]。也有研究通过物理、化学等手段,将PAA与其他聚合物进行交联。将PR及PAA进行化学交联,用作Py-c-SiO负极黏结剂,可以有效地抑制硅颗粒的膨胀,提高电极的可恢复能力,延长电池的使用寿命[70]。利用紫外交联制备PAA-BP黏结剂[71],图 4为PAA-BP黏结剂的合成方法及其适应硅颗粒体积膨胀示意图。也有研究采用物理化学交联剂制备含有一定物理化学交联的c-LiPAACA-Laponite黏结剂[72]。
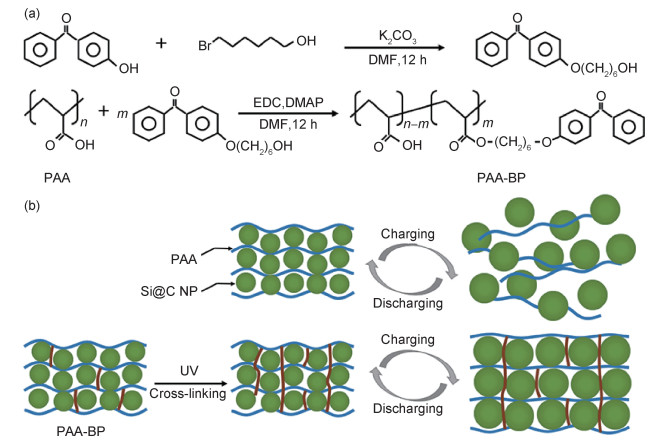
|
图 4 PAA-BP黏结剂的合成方法及其适应硅颗粒体积膨胀示意图[71] (a)功能化PAA-BP黏结剂的合成方法;(b)PAA-BP黏结剂适应充放电循环期间硅阳极的体积膨胀示意图 Fig. 4 Synthesis of PAA-BP binder and schematic diagram of PAA-BP binder accommodating Si particle volume expansion[71] (a)synthetic approach for the preparation of the functionalized PAA-BP binder; (b)schematic diagram of PAA-BP binder accommodating the volume expansion of the Si anode during charging and discharging progress |
将PAA与其他聚合物共混、交联等处理,均是通过改变PAA的交联度或与其他聚合物产生协同效应来提高锂电池的电性能,并没有涉及PAA自身分子链结构的改变,有研究采用接枝等手段,减少或增加PAA中相应官能团数量或引入其他有效的弹性链段等,来改善PAA自身结构,以更好地用于电池浆料中,如PVA-g-PAA[28],NaPAA-g-CMC[47]及PNA-NaPAA-g-CMC[48]等。将PVA与丙烯酸单体(AA) 于Ar气氛中进行引发,接枝聚合,制备PVA-g-PAA黏结剂用于硅负极。相较于纯PAA电极(0.91 N·cm-1),得到的PVA-g-10PAA(1 g PVA/10 g PAA,下同)的电极黏附强度最高,为1.14 N·cm-1,这可能归因于其接枝聚合物网络,在Si颗粒与黏结剂之间引入更具活性的交联位点,且因锚定作用及均匀分散的Si粒子和导电添加剂,而维持一个牢固的机械和电气网络[28]。在硅负极中,原位接枝聚合制备的NaPAA-g-CMC黏结剂,使得黏结剂与活性材料的接触位点增加,黏附力增强,电极的平均剥离力也比使用纯PAA (2.2 N)的更高,达2.7 N[47]。
3 首次库仑效率PAA黏结剂具有较低的溶胀性,且含有大量的羧基,可以与硅材料表面的羟基形成氢键及共价键,显著提高黏附力,抑制体积膨胀,有利于活性材料表面形成较为稳定的薄的人工SEI层[22],改性PAA黏结剂可以更好地发挥这些作用。
在体积膨胀较大的硅负极中,采用GA改性的PAA黏结剂(PAA-GA)的电极表现出3739 mAh·g-1 的初始放电容量,高于同等条件下的CMC和PAA,但其首次库仑效率(ICE)为86.5%,与二者相近[27]。用PVA-g-PAA黏结剂的电极相较于PVA,PAA及CMC表现出更高的首次库仑效率,尤其当接枝聚合物中PAA的添加量为10 g时效率最高,约82.4%。经1000个循环后,仍能保持1315.8 mAh·g-1的放电容量和40.3%的容量保持率[28]。使用PDA-PAA-PEO三嵌段共聚物与PAA-PEO-PAA黏结剂的电极分别表现出74%和80%的首次库仑效率,而相同条件下使用PVDF仅68%[29]。
此外,对活性材料进行表面处理后配合适当的黏结剂使用,也是较好的提高库仑效率的方法。有人用不同质量比的TA表面处理的SiSMPs(SiSMPs@TA)与PAA黏结剂搭配使用,搭配PAA黏结剂的SiSMPs@TA(任意比例TA处理)电极的初始放电容量和首次库仑效率均高于未处理的SiSMPs电极[58]。交联PAA-GL黏结剂与Si@SiO2配合使用,获得3659.4 mAh·g-1的初始放电容量,远高于CMC及PAA,首次库仑效率也最高,为88.87%[61]。
在硅碳负极中,使用PAA-Rosin(质量比1∶1,下同)黏结剂的电池的初始库仑效率为86.6%,明显高于使用PAA的电池(85.7%)和使用Rosin的电池(60.0%)[66]。与Void@SiOx@C搭配,相较于PVDF和PAA,采用原位交联c-PAA-DS黏结剂的电池获得更高的首次库仑效率61.3%[73]。处理过后的负极活性材料α-MnO2与PAANa黏结剂配合使用后,库仑效率接近100%,相同条件下使用PVDF仅71%[65]。
PAA黏结剂用于正极时,使得电池的库仑效率也颇为优异。采用PAA-PVA(2∶1)黏结剂的LiFePO4/C正极在0.2 C时得到的初始库仑效率较PAA和PVA高得多,为98.5%[33]。使用PAALi黏结剂的LRMO正极的初始放电容量和首次库仑效率略低于PVDF基电极,但在3次循环后,有明显的激活过程,容量增加[74]。表 1为不同PAA黏结剂较PVDF对不同电极的首次库仑效率的影响,表 2是不同改性PAA黏结剂较纯PAA对不同电极首次库仑效率的影响。
结果表明,采用纯PAA黏结剂的电极较PVDF有更好的首次库仑效率,有利于在活性材料表面形成SEI膜。而采用改性PAA黏结剂的电极的首次库仑效率更高,这说明,对PAA进行物理化学改性,可改变PAA的部分结构及官能团,能够促使更稳定的SEI膜的形成,从而提高黏结剂对活性材料体积变化的承受能力,抑制电解液对电极的腐蚀,更好地提高电池的循环稳定性。
4 循环性能合适的黏结剂具有适当的黏性及力学性能,从而提供良好的黏附力,抑制极片在充放电过程中因活性材料体积变化而发生形变,这是提高电池循环稳定性的关键指标之一。
ACC/PAA作为SiSMPs负极黏结剂,由于其高交联网络和机械韧性,能够承受SiSMPs剧烈的体积膨胀,在100次循环后仍可保持75%的容量且电极表面依旧平整,相比于ACC-PAA黏结剂,CMC,SA和PVDF基容量保持率仅35%,24%和0.6%,电极表面也出现较大的裂纹[57]。有研究者用接枝聚合物NaPAA-g-CMC作硅负极黏结剂使用,也表现出了较为优异的循环性能。Si负载为0.19 mg·cm-2时,100次循环后容量保持率达87%,同等条件下使用PAA,CMC和NaPAA-mix-CMC黏结剂的锂电池仅为39%,45.5%和43%。当Si负载为0.45 mg·cm-2时,使用NaPAA-g-CMC的锂电池100次循环后容量保持率可达79.3%,即使达1.3 mg·cm-2的高质量Si负载,也能在50次循环后保持80%的容量[47]。PAA-PAI作为Si/C负极黏结剂使用时,其锂电池具有1120.9 mAh·g-1的可逆容量,且在100次循环后保持有66.2%,远高于PAA和PAI,300次循环后仍保有47.0%,而相同条件下,PAA和PAI仅38.2%和30.1%[63]。
PAA-CMC黏结剂用于Co3O4负极时,由于PAA和CMC的协同作用,在电流密度200 mA·g-1下循环150次后,容量保持率可达86.7%,而PAA和CMC仅有1%和66.8%[64]。
由于Si表面的官能团(羧基和邻苯二酚)与羟基之间的强相互作用,使得PDA-PAA-PEO黏结剂在硅负极中具有很强的黏结力,故而其锂电池在0.5 C下循环200次后仍具有1597 mAh·g-1的可逆容量,远远高于PAA-PEO-PAA的1205 mAh·g-1。相比较而言,相同条件下使用PVDF电池,循环50次后仅有40 mAh·g-1的容量[29]。此外,PNA-NaPAA-g-CMC用作硅负极黏结剂,当Si质量负载为0.6 mg·cm-2时,该锂电池250次循环后仍具有2220 mAh·g-1的高放电容量,容量保持率达94%,而CMC和PVDF经100次循环后容量仅保留45.5%和20%。即使Si质量负载增加到0.9 mg·cm-2时,使用PNA-NaPAA-g-CMC的锂电池也能在100次循环后仍保有90%的容量[48]。
以c-PAA-DS为黏结剂,Void@SiOx@C为负极活性材料的锂电池,在500 mA·g-1下循环500次后容量并未出现衰减,反而有所增加,而PAA和PVDF仅保留71%和24%的容量。且c-PAA-DS黏结剂在高负载活性材料情况下,长期循环后,仍出现容量略增现象。这可能是由于c-PPA-DS黏结剂的黏附力强,加强了Void@SiOx@C与导电剂的连接,从而维持极片在充放电过程中的稳定性,提高循环性能[73]。
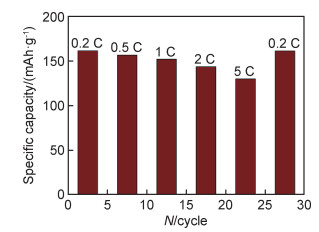
纯PAA作为LRMO正极黏结剂时,其电极在100 mA·g-1电流密度下循环100次后保持97.7%的容量,在200 mA·g-1电流密度下循环500次后仍保持88%的容量[75]。在LiFePO4/C正极中,采用PAA-PVA黏结剂的锂电池较PAA和PVA具有更好的循环稳定性。PAA-PVA(1∶1)在循环300次后具有96.8%的容量保持率,PAA-PVA(2∶1)甚至达到100.0%,而同等条件下的PAA和PVA仅88.8%和82.0%[33],图 5是采用PAA-PVA(2∶1)黏结剂的LiFePO4/C正极的倍率性能图。表 3为不同改性PAA黏结剂在不同电极中多次循环后的容量保持率, 表 4是不同改性PAA黏结剂在不同电极中长期循环后的容量保持率。
| Binder | Capacity retention/% | Growth rate/% | N/cycle | |
| Target group | Control group | |||
| PAA-GA[27] | 81 | 69.6 | 14.07 | 285 at 0.2 C |
| PAA-10% PANI[67] | 56.5 | 11.3 | 80 | 300 at 1 C |
| PAA-PVA (2∶1)[33] | 100.0 | 88.8 | 11.2 | 300 at 5 C |
| c-PAA-DS [73] | Increased | 71 | >29 | 500 at 500 mA·g-1 |
| 1000 at 5 A·g-1 | ||||
| PVA-g-10PAA[28] | 40.3 | 1000 at 400 mA·g-1 | ||
相较于PVDF,具有大量羧基基团的PAA黏结剂对正负极片有更高的黏附力,因此也使得电池具有更高的循环稳定性,减小容量损失。但PAA因其分子链刚性较大,而对电池循环性能的提高有一定的限制。采用改性PAA黏结剂的电池的循环性能较纯PAA黏结剂来说有较大的提高,容量保持率明显提升,且在长期循环后,仍能保持较高的容量。这可能归因于改性的PAA黏结剂中引入了部分柔性链段,均衡了极性官能团数量,而具有更为合适的力学性能和黏附力,更利于在充放电过程中,维持电极的均匀平整。
5 阻抗性能锂离子电池阻抗一般由三部分组成:高频区的欧姆电阻,中频区的电荷传递阻抗(RCT)[76]及低频区的Warburg阻抗[5]。合适的黏结剂在提供良好的黏附力的同时,也可以提供较好的电子网络,提高电子的传输和锂离子的扩散性能。且可以在活性材料表面形成稳定的SEI膜,从而降低阻抗,提高电池的总体电化学性能。
采用CS-PAA和CS-PAANa作为硅负极的黏结剂,循环10次后电极的电荷传递阻抗有所增加,这可能是由于电接触损耗和应力集中引起的锂离子扩散的阻碍,但使用CS-PAA和CS-PAANa黏结剂的锂电池的阻抗值远小于PVDF,且对应的锂离子扩散系数(DLi)也更大,分别为2.91×10-15 cm2·s-1和5.41×10-15 cm2·s-1。这也表明能够形成化学键和非晶聚集的黏结剂CS-PAA和CS-PAANa可以促进电极在充放电过程中的稳定性,减小阻抗,提高锂离子扩散速率[76]。此外,电解液的分解在电池老化或容量衰减中起着至关重要的作用,可能在循环过程中生成HF副产物,降低电池的使用寿命,间接影响锂离子的扩散而影响电池的使用性能。为了抑制HF的生成而破坏电池的使用性能,有人将强螯剂EDTA引入PAA黏结剂(质量比9∶1,下同),用于硅负极,得到更小的RCT和更高的DLi,分别为16.1 Ω和3.92×10-14 cm2·s-1[41]。同样,将PAA-Rosin用于Si/C负极,其电极的界面阻抗RIN为18.57 Ω,而纯Rosin的RIN为26.89 Ω。这说明PAA-Rosin黏结剂有利于在Si/C电极上形成稳定的SEI层,减小阻抗,提高锂离子扩散速率[66]。
在ZnMoO4·0.8H2O负极中,用CMC-PAA混合黏结剂制成的锂电池的阻抗为25.9 Ω,相较于使用PVDF(122.7 Ω)和CMC(54.5 Ω),CMC-PAA的锂电池的阻抗明显小得多,有利于锂离子扩散,DLi为8.7×10-9 cm2·s-1,而相同条件下使用PVDF仅5.0×10-12 cm2·s-1 [5]。
当PAA-PVA黏结剂用于LiFePO4/C正极时,相比于PAA或PVA,当PAA-PVA为2∶1时获得最低的电荷传递阻抗18.0 Ω和最高的锂离子扩散系数6.8×10-12 cm2·s-1 [33]。
在硅-石墨负极中,使用PAA-PAA/PMA(质量比1∶1,下同)黏结剂电极的电解质界面阻抗RSEI和电荷传递阻抗RCT分别为2.5 Ω和52.8 Ω。相较于PAA(7.3,156.8 Ω)和PAA/PMA(3.9,140.0 Ω),使用PAA-PAA/PMA电极的阻抗明显更低。这是由于电池充放电过程中,硅-石墨复合材料的粉化使得表面被暴露出来,会导致新SEI层形成,这会大大增加电极阻抗,从而减小锂离子的扩散速率,而PAA-PAA/PMA对硅-石墨复合材料粉化的抑制作用,保证更好的电极均匀性,有利于活性材料表面形成稳定的SEI膜,降低阻抗[62]。由于PEO嵌段具有较高的离子电导率,且PDA-PAA-PEO具有强的内聚力,可以使得电极分布更加均匀,从而维持极片在充放电过程中的完整性,减小电极的电荷传递阻抗。实验证明,采用PDA-PAA-PEO黏结剂的硅负极的阻抗为50 Ω,远小于使用PVDF的130 Ω[29]。表 5是不同改性PAA黏结剂在不同电极中的阻抗数据表,表 6为不同改性PAA黏结剂较纯PAA在不同电极中的锂离子扩散系数。
将改性后的PAA黏结剂用于锂电池正负极,可以更好地抑制活性材料的体积膨胀,形成更稳定的薄SEI层,有效地阻隔电解液对活性材料的腐蚀,降低电极的阻抗,尤其是电荷传递阻抗,提高锂离子扩散速率,这对于电池使用性能的提高具有至关重要的作用。
6 结束语黏结剂作为电极中占比较少的部分,不仅起到黏附作用,也可间接增加电池比容量,提高循环稳定性。PAA黏结剂因羧基的存在而具有良好的黏附力,与其他聚合物进行共混、聚合后,可以与之产生协同作用,利于电池电性能的提升。但PAA自身的官能团单一,且链刚性过大,限制了极片的长期循环稳定性,且PAA本身不导电,不利于电池电导率的提高。故而PAA作为锂电池正负极用黏结剂,对电池电化学性能的提升仍然还有潜力。
在对PAA类黏结剂未来的研究中,应考虑弹性结构、极性基团及物理化学接触点数量等因素,使PAA黏结剂能够更好地用于体积膨胀较大的硅负极;探索使用导电聚合物或导电基团的PAA黏结剂对活性材料占比的影响,考虑黏结剂的聚合物结构及电解液与导电性差、振实密度及锂离子扩散速率低的LiFePO4电极性能的关联;因富锂锰氧化物(LRMOs)正极材料的循环稳定性较差,应考虑改性PAA黏结剂能否在其表面形成致密表面保护膜,并能抑制不可逆的过渡金属转移。这些研究难点都将是未来提升各类锂电池性能的关键。此外,在提高锂电池性能的同时,PAA黏结剂改性工艺的难易、成本高低以及环保性也是未来研究需要考虑的。
| [1] |
LIU W, SONG M S, KONG B, et al. Flexible and stretchable energy storage: recent advances and future perspectives[J]. Advanced Materials, 2017, 29(1): 1603436. DOI:10.1002/adma.201603436 |
| [2] |
GU S J, CUI Y Y, WEN K H, et al. 3-cyano-5-fluorobenzenzboronic acid as an electrolyte additive for enhancing the cycling stability of Li1.2Mn0.54Ni0.13Co0.13O2 cathode at high voltage[J]. Journal of Alloys and Compounds, 2020, 829: 154491. DOI:10.1016/j.jallcom.2020.154491 |
| [3] |
WU Z Y, DENG L, LI J T, et al. Multiple hydrogel alginate binders for Si anodes of lithium-ion battery[J]. Electrochimica Acta, 2017, 245: 371-378. DOI:10.1016/j.electacta.2017.05.094 |
| [4] |
MA S S, LIN H, YANG L Y, et al. High thermal stability and low impedance polypropylene separator coated with aluminum phosphate[J]. Electrochimica Acta, 2019, 320: 134528. DOI:10.1016/j.electacta.2019.07.039 |
| [5] |
FEI J, SUN Q Q, CUI Y L, et al. Sodium carboxyl methyl cellulose and polyacrylic acid binder with enhanced electrochemical properties for ZnMoO4·0.8H2O anode in lithium ion batteries[J]. Journal of Electroanalytical Chemistry, 2017, 804: 158-164. DOI:10.1016/j.jelechem.2017.09.061 |
| [6] |
NGUYEN Q D, CHUNG K. Frictional properties of polymer binders for Li-ion batteries[J]. Applied Physics Letters, 2020, 116(6): 061604. DOI:10.1063/1.5141495 |
| [7] |
MOCHIZUKI T, AOKI S, HORIBA T, et al. "Natto" binder of poly-γ-glutamate enabling to enhance silicon/graphite composite electrode performance for lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 6343-6355. |
| [8] |
VERDIERA N, KHAKANIA S E, LEPAGE D, et al. Polyacrylonitrile-based rubber (HNBR) as a new potential elastomeric binder for lithium-ion battery electrodes[J]. Journal of Power Sources, 2019, 440: 227111. DOI:10.1016/j.jpowsour.2019.227111 |
| [9] |
FONT F, PROTASB B, RICHARDSON G, et al. Binder migration during drying of lithium-ion battery electrodes: modelling and comparison to experiment[J]. Journal of Power Sources, 2018, 393: 177-185. DOI:10.1016/j.jpowsour.2018.04.097 |
| [10] |
MAZOUZI D, KARKAR Z, HERNANDEZ C R, et al. Critical roles of binders and formulation at multiscales of silicon-based composite electrodes[J]. Journal of Power Sources, 2015, 280: 533-549. DOI:10.1016/j.jpowsour.2015.01.140 |
| [11] |
TANG R X, MA L, ZHANG Y, et al. A flexible and conductive binder with strong adhesion for high performance silicon-based lithium-ion battery anode[J]. ChemElectroChem, 2020, 7(9): 1992-2000. DOI:10.1002/celc.201902152 |
| [12] |
LING M, XU Y N, ZHAO H, et al. Dual-functional gum Arabic binder for silicon anodes in lithium ion batteries[J]. Nano Energy, 2015, 12: 178-185. DOI:10.1016/j.nanoen.2014.12.011 |
| [13] |
李娟娟. 基于聚丙烯酸的锂离子电池硅负极黏结剂的研究[D]. 广州: 华南理工大学, 2019. LI J J.Investigation of polyacrylic acid-based binders for silicon anodes in lithium-ion batteries[D].Guangzhou: South China University of Technology, 2019. |
| [14] |
QIAN J, WIENER C G, ZHU Y, et al. Swelling and plasticization of polymeric binders by Li-containing carbonate electrolytes using quartz crystal microbalance with dissipation[J]. Polymer, 2018, 143: 237-244. DOI:10.1016/j.polymer.2018.04.021 |
| [15] |
YI D W, CUI X M, LI N L, et al. Enhancement of electrochemical performance of LiFePO4@C by Ga coating[J]. ACS Omega, 2020, 5(17): 9752-9758. DOI:10.1021/acsomega.9b04165 |
| [16] |
YOU M L, HUANG X K, LIN M, et al. Preparation of LiCo-MnO4 assisted by hydrothermal approach and its electrochemical performance[J]. American Journal of Engineering and Applied Sciences, 2016, 9(2): 396-405. DOI:10.3844/ajeassp.2016.396.405 |
| [17] |
WU H W, PANG X F, BI J X, et al. Cellulose nanofiber assisted hydrothermal synthesis of Ni-rich cathode materials with high binding particles for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2020, 829: 154571. DOI:10.1016/j.jallcom.2020.154571 |
| [18] |
MYUNG S, MAGLIA F, PARK K J, et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives[J]. ACS Energy Letters, 2017, 2(1): 196-223. DOI:10.1021/acsenergylett.6b00594 |
| [19] |
YOON C S, PARK K J, KIM U H, et al. High-energy Ni-rich Li[NixCoyMn1-x-y]O2 cathodes via compositional partitioning for next-generation electric vehicles[J]. Chemical of Materials, 2017, 29(24): 10436-10445. DOI:10.1021/acs.chemmater.7b04047 |
| [20] |
LIU J, ZHANG Q, ZHANG T, et al. A robust ion-conductive biopolymer as a binder for Si anodes of lithium-ion batteries[J]. Advanced Functional Materials, 2015, 25(23): 3599-3605. DOI:10.1002/adfm.201500589 |
| [21] |
GENDENSUREN B, OH E. Dual-crosslinked network binder of alginate with polyacrylamide for silicon/graphite anodes of lithium ion battery[J]. Journal of Power Sources, 2018, 384: 379-386. DOI:10.1016/j.jpowsour.2018.03.009 |
| [22] |
岳丽萍, 韩鹏献, 姚建华, 等. 锂离子电池硅基负极黏结剂研究进展[J]. 电池工业, 2017, 21(1): 31-44. YUE L P, HAN P X, YAO J H, et al. Advances of binder for silicone-based anode in lithium ion batteries[J]. Chinese Battery Industry, 2017, 21(1): 31-44. |
| [23] |
李延良, 夏清华, 张杰. 水性黏结剂在硅基负极中的应用研究进展[J]. 广东化工, 2019, 46(13): 106-107. LI Y L, XIA Q H, ZHANG J. Application of water-soluble binder in Si-based anodes[J]. Guangdong Chemical Industry, 2019, 46(13): 106-107. |
| [24] |
LEE J H, PAIK U, HACKLEY V A, et al. Effect of poly(acrylic acid) on adhesion strength and electrochemical performance of natural graphite negative electrode for lithium-ion batteries[J]. Journal of Power Sources, 2006, 161(1): 612-616. DOI:10.1016/j.jpowsour.2006.03.087 |
| [25] |
MARONIA F, GABRIELLIA S, PALMIERI A, et al. High cycling stability of anodes for lithium-ion batteries based on Fe3O4nanoparticles and poly(acrylic acid) binder[J]. Journal of Power Sources, 2016, 332: 79-87. DOI:10.1016/j.jpowsour.2016.09.106 |
| [26] |
LI J, LE D B, FERGUSON P P, et al. Lithium polyacrylate as a binder for tin-cobalt-carbon negative electrodes in lithium-ion batteries[J]. Electrochimica Acta, 2010, 55(8): 2991-2995. DOI:10.1016/j.electacta.2010.01.011 |
| [27] |
LI J J, ZHANG G Z, YANG Y, et al. Glycinamide modified polyacrylic acid as high-performance binder for silicon anodes in lithium-ion batteries[J]. Journal of Power Sources, 2018, 406: 102-109. DOI:10.1016/j.jpowsour.2018.10.057 |
| [28] |
HE J R, ZHANG L Z. Polyvinyl alcohol grafted poly(acrylic acid) as water-soluble binder with enhanced adhesion capability and electrochemical performances for Si anode[J]. Journal of Alloys and Compounds, 2018, 763: 228-240. DOI:10.1016/j.jallcom.2018.05.286 |
| [29] |
LV L, LOU H M, XIAO Y L, et al. Synthesis of triblock copolymer polydopamine-polyacrylic-polyoxyethylene with excellent performance as a binder for silicon anode lithium-ion batteries[J]. RSC Advances, 2018, 8(9): 4604-4609. DOI:10.1039/C7RA13524F |
| [30] |
KOMABA S, OZEKI T, OKUSHI K. Functional interface of polymer modified graphite anode[J]. Journal of Power Sources, 2009, 189(1): 197-203. DOI:10.1016/j.jpowsour.2008.09.092 |
| [31] |
UI K, TOWADA J, AGATSUMA S, et al. Influence of the binder types on the electrochemical characteristics of natural graphite electrode in room-temperature ionic liquid[J]. Journal of Power Sources, 2011, 196(8): 3900-3905. DOI:10.1016/j.jpowsour.2010.12.007 |
| [32] |
CAI Z P, LIANG Y, LI W S, et al. Preparation and performances of LiFePO4 cathode in aqueous solvent with polyacrylic acid as a binder[J]. Journal of Power Sources, 2009, 189(1): 547-551. DOI:10.1016/j.jpowsour.2008.10.040 |
| [33] |
SUN J C, REN X, LI Z F, et al. Effect of poly(acrylic acid)/poly(vinyl alcohol) blending binder on electrochemical performance for lithium iron phosphate cathodes[J]. Journal of Alloys and Compounds, 2019, 783: 379-386. DOI:10.1016/j.jallcom.2018.12.197 |
| [34] |
ZHANG Z A, ZENG T, LAI Y Q, et al. A comparative study of different binders and their effects on electrochemical properties of LiMn2O4 cathode in lithium ion batteries[J]. Journal of Power Sources, 2014, 247: 1-8. DOI:10.1016/j.jpowsour.2013.08.051 |
| [35] |
PARIKH P, SINA M, BANERJEE A, et al. Role of polyacrylic acid (PAA) binder on the solid electrolyte interphase in silicon anodes[J]. Chemistry of Materials, 2019, 31(7): 2535-2544. DOI:10.1021/acs.chemmater.8b05020 |
| [36] |
HU B, SHKROB I A, ZHANG S, et al. The existence of optimal molecular weight for poly(acrylic acid) binders in silicon/graphite composite anode for lithium-ion batteries[J]. Journal of Power Sources, 2018, 378: 671-676. DOI:10.1016/j.jpowsour.2017.12.068 |
| [37] |
KARKAR Z, GUYOMARD D, ROUE L, et al. A comparative study of polyacrylic acid (PAA) and carboxymethyl cellulose (CMC) binders for Si-based electrodes[J]. Electrochimica Acta, 2017, 258: 453-466. DOI:10.1016/j.electacta.2017.11.082 |
| [38] |
LI C, SHI T F, YOSHITAKE H, et al. Improved performance in micron-sized silicon anodes by in-situ polymerization of its acrylic acid-based slurry[J]. Journal of Materials Chemistry A, 2016, 4(43): 16982-16991. DOI:10.1039/C6TA05650D |
| [39] |
郭容男, 韩伟强. 极性聚合物黏结剂的结构和物性对锂离子电池的影响[J]. 无机材料学报, 2019, 24(10): 1021-1029. GUO R N, HAN W Q. Effects of structure and properties of polar polymeric binders on lithium-ion batteries[J]. Journal of Inorganic Materials, 2019, 24(10): 1021-1029. |
| [40] |
PORCHER W, CHAZELLE S, BOULINEAU A, et al. Understanding polyacrylic acid and lithium polyacrylate binder behavior in silicon based electrodes for Li-ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(14): 3633-3640. DOI:10.1149/2.0821714jes |
| [41] |
LEE S Y, CHOI Y, HONG K S, et al. Influence of EDTA in poly(acrylic acid) binder for enhancing electrochemical performance and thermal stability of silicon anode[J]. Applied Surface Science, 2018, 447: 442-451. DOI:10.1016/j.apsusc.2018.04.004 |
| [42] |
KOMABA S, SHIMOMURA K, YABUUCHI N, et al. Study on polymer binders for high-capacity SiO negative electrode of Li-ion batteries[J]. Journal of Physical Chemistry C, 2011, 115(27): 13487-13495. DOI:10.1021/jp201691g |
| [43] |
曾涛. 聚丙烯酸黏结剂在锂离子电池正极中的应用研究[D]. 长沙: 中南大学, 2013. ZENG T.Study on polyacrylic acid as binder of cathode in lithium ion battery[D].Changsha: Central South University, 2013. |
| [44] |
KOVALENKO I, ZDYRKO B, MAGASINSKI A, et al. A major constituent of brown algae for use in high-capacity Li-ion batteries[J]. Science, 2011, 334(6052): 75-79. DOI:10.1126/science.1209150 |
| [45] |
OBROVAC M N, CHEVRIER V. Alloy negative electrodes for Li-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11444-11502. DOI:10.1021/cr500207g |
| [46] |
ZHENG Z N, GAO X, LUO Y W. Influence of copolymer chain sequence on electrode latex binder for lithium-ion batteries[J]. Colloid and Polymer Science, 2019, 297(10): 1287-1299. DOI:10.1007/s00396-019-04548-9 |
| [47] |
WEI L M, CHEN C X, HOU Z Y, et al. Poly(acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries[J]. Scientific Reports, 2016, 6(1): 19583. DOI:10.1038/srep19583 |
| [48] |
WEI L M, HOU Z Y. High performance polymer binders inspired by chemical finishing of textiles for silicon anodes in lithium ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(42): 22156-22162. DOI:10.1039/C7TA05195F |
| [49] |
BIE Y, YANG J, NULI Y, et al. Natural karaya gum as an excellent binder for silicon-based anodes in high-performance lithium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(5): 1919-1924. DOI:10.1039/C6TA09522D |
| [50] |
BIE Y, YANG J, LIU X L, et al. Polydopamine wrapping silicon cross-linked with polyacrylic acid as high-performance anode for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(5): 2899-2904. |
| [51] |
SHIN D, PARK H, PAIK U. Cross-linked poly(acrylic acid)-carboxymethyl cellulose and styrene-butadiene rubber as an efficient binder system and its physicochemical effects on a high energy density graphite anode for Li-ion batteries[J]. Electrochemistry Communications, 2017, 77: 103-106. DOI:10.1016/j.elecom.2017.02.018 |
| [52] |
LEE S H, LEE J H, NAM D H, et al. Epoxidized natural rubber/chitosan network binder for silicon anode in lithium-ion battery[J]. ACS Applied Materials & Interfaces, 2018, 10(19): 16449-16457. |
| [53] |
XU Z X, YANG J, ZHANG T, et al. Silicon microparticle anodes with self-healing multiple network binder[J]. Joule, 2018, 2(5): 950-961. DOI:10.1016/j.joule.2018.02.012 |
| [54] |
WANG L, LIU T F, PENG X, et al. Highly stretchable conductive glue for high-performance silicon anodes in advanced lithium-ion batteries[J]. Advanced Functional Materials, 2018, 28(3): 1704858. DOI:10.1002/adfm.201704858 |
| [55] |
ZENG W W, WANG L, PENG X, et al. Enhanced ion conductivity in conducting polymer binder for high-performance silicon anodes in advanced lithium-ion batteries[J]. Advanced Energy Materials, 2018, 8(11): 1702314. DOI:10.1002/aenm.201702314 |
| [56] |
CHOI S, KWON T, COSKUN A, et al. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries[J]. Science, 2017, 357(6348): 279-283. DOI:10.1126/science.aal4373 |
| [57] |
TIAN M, CHEN X, SUN S T, et al. A bioinspired high-modulus mineral hydrogel binder for improving the cycling stability of microsized silicon particle-based lithium-ion battery[J]. Nano Research, 2019, 12(5): 1121-1127. DOI:10.1007/s12274-019-2359-y |
| [58] |
TIAN M, WU P Y. Nature plant polyphenol coating silicon submicroparticle conjugated with polyacrylic acid for achieving a high-performance anode of lithium-ion battery[J]. ACS Applied Energy Materials, 2019, 2(7): 5066-5073. DOI:10.1021/acsaem.9b00734 |
| [59] |
ZHANG J F, REN T, NAYAKA G P, et al. Design of polydopamine-encapsulation multiporous MnO cross-linked with polyacrylic acid binder for superior lithium ion battery anode[J]. Journal of Alloys and Compounds, 2019, 783: 341-348. DOI:10.1016/j.jallcom.2018.12.356 |
| [60] |
HWANG J, KIM H, SUN Y. High performance potassium-sulfur batteries based on a sulfurized polyacrylonitrile cathode and polyacrylic acid binder[J]. Journal of Materials Chemistry A, 2018, 6(30): 14587-14593. DOI:10.1039/C8TA03135E |
| [61] |
SUN S, HE D L, LI P, et al. Improved adhesion of cross-linked binder and SiO2-coating enhances structural and cyclic stability of silicon electrodes for lithium-ion batteries[J]. Journal of Power Sources, 2020, 454: 227907. DOI:10.1016/j.jpowsour.2020.227907 |
| [62] |
YIM T, CHOI S J, PARK J, et al. The effect of an elastic functional group in a rigid binder framework of silicon-graphite composites on their electrochemical performance[J]. Physical Chemistry Chemical Physics, 2015, 17(4): 2388-2393. DOI:10.1039/C4CP04723K |
| [63] |
YIM T, CHOI S J, JO Y N, et al. Effect of binder properties on electrochemical performance for silicon-graphite anode: method and application of binder screening[J]. Electrochimica Acta, 2014, 136: 112-120. DOI:10.1016/j.electacta.2014.05.062 |
| [64] |
YANG X F, ZHANG H M, MING H, et al. Aqueous binder effects of poly(acrylic acid) and carboxy methylated cellulose on anode performance in lithium-ion batteries[J]. New Journal of Chemistry, 2019, 43(32): 12555-12562. DOI:10.1039/C9NJ02078K |
| [65] |
AN Y L, FENG J K, CI L J, et al. MnO2 Nanotubes with a water soluble binder as high performance sodium storage materials[J]. RSC Advances, 2016, 6(105): 103579-103584. DOI:10.1039/C6RA20706E |
| [66] |
CHOI S J, YIM T, CHO W, et al. Rosin-embedded poly(acrylic acid) binder for silicon/graphite negative electrode[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(12): 6362-6370. |
| [67] |
LEE K, LIM S, TRON A, et al. Polymeric binder based on PAA and conductive PANI for high performance silicon-based anodes[J]. RSC Advances, 2016, 6(103): 101622-101625. DOI:10.1039/C6RA23805J |
| [68] |
ZHENG M Y, CAI X M, TAN Y F, et al. A high-resilience and conductive composite binder for lithium-sulfur batteries[J]. Chemical Engineering Journal, 2020, 389: 124404. DOI:10.1016/j.cej.2020.124404 |
| [69] |
JIANG S S, HU B, SHI Z X, et al. Re-engineering poly(acrylic acid) binder toward optimized electrochemical performance for silicon lithium-ion batteries: branching architecture leads to balanced properties of polymeric binders[J]. Advanced Functional Materials, 2020, 30(10): 1908558. DOI:10.1002/adfm.201908558 |
| [70] |
CHO Y M, KIM J, ELABD A, et al. A pyrene-poly(acrylic acid)-polyrotaxane supramolecular binder network for high-performance silicon negative electrodes[J]. Advanced Materials, 2019, 31(51): 1905048. DOI:10.1002/adma.201905048 |
| [71] |
PARK Y, LEE S, KIM S, et al. A photo-cross-linkable polymeric binder for silicon anodes in lithium ion batteries[J]. RSC Advances, 2013, 3(31): 12625-12630. DOI:10.1039/c3ra42447b |
| [72] |
GUO R N, WANG J L, ZHANG S L, et al. Multifunctional cross-linked polymer-Laponite nanocomposite binder for lithium-sulfur batteries[J]. Chemical Engineering Journal, 2020, 388: 124316. DOI:10.1016/j.cej.2020.124316 |
| [73] |
HE D L, LI P, WANG W, et al. Collaborative design of hollow nanocubes, in situ cross-linked binder, and amorphous void@SiOx@C as a three-pronged strategy for ultrastable lithium storage[J]. Small, 2019, 16(5): 1905736. |
| [74] |
SONG J H, FENG Z H, WANG Y, et al. Suppressed volume variation of optimized SiOx/C anodes with PAA-based binders for advanced lithium-ion pouch cells[J]. Solid State Ionics, 2019, 343: 115070. DOI:10.1016/j.ssi.2019.115070 |
| [75] |
YANG J S, LI P, ZHONG F P, et al. Suppressing voltage fading of Li-rich oxide cathode via building a well-protected and partially-protonated surface by polyacrylic acid binder for cycle-stable Li-ion batteries[J]. Advanced Energy Materials, 2020, 10(15): 1904264. DOI:10.1002/aenm.201904264 |
| [76] |
GAO Y, QIU X T, WANG X L, et al. Chitosan-g-poly(acrylic acid) copolymer and its sodium salt as stabilized aqueous binders for silicon anodes in lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(19): 16274-16283. |
 2021, Vol. 49
2021, Vol. 49