文章信息
- 张舒研, 高洋洋, 张志彬, 梁秀兵, 王立忠
- ZHANG Shu-yan, GAO Yang-yang, ZHANG Zhi-bin, LIANG Xiu-bing, WANG Li-zhong
- 高熵非晶合金耐腐蚀性能研究进展
- Research progress in corrosion resistance of high-entropy metallic glasses
- 材料工程, 2021, 49(1): 44-54
- Journal of Materials Engineering, 2021, 49(1): 44-54.
- http://dx.doi.org/10.11868/j.issn.1001-4381.2020.000254
-
文章历史
- 收稿日期: 2020-03-25
- 修订日期: 2020-09-12
2. 军事科学院 国防科技创新研究院, 北京 100071
2. National Innovation Institute of Defense Technology, Academy of Military Sciences of the PLA of China, Beijing 100071, China
高熵非晶合金(high-entropy metallic glasses, HE-MGs)是近年来发展起来的一种新型材料,中科院物理所汪卫华院士课题组于2011年首次提出了高熵非晶合金的概念,同时制备出具有无序结构并能在室温下表现出类似于高分子热塑性变形行为的块体高熵非晶合金Zn20Ca20Sr20Yb20(Li0.55Mg0.45)20[1]。随后不久,日本东北大学的Takeuchi等制备出同时含有金属元素与非金属元素的高熵非晶合金Pd20Pt20Cu20Ni20P20[2]。它综合利用了高熵合金与非晶合金的设计理念,兼具两类合金各自的性能特点,所以其开发与应用备受瞩目。近10年来,国内外学者对该类材料进行了初步探究,加深了对其基本性能的理解,拓宽了高熵非晶合金作为一种功能材料的使用范围。本文重点阐述了高熵非晶合金的耐腐蚀性,旨在提出面向海洋极端环境服役的耐腐蚀性材料的成分设计理论及制备方法,以满足国家实施海洋发展战略对高性能大型装备防护材料的需求。
1 高熵非晶合金的含义与体系概述非晶合金(metallic glasses, MGs)在20世纪60年代由美国的Duwez教授通过熔体旋淬法首次获得[3],这种材料是在超急冷凝固条件下形成的;其原子排列呈现长程无序、短程有序的特点,表现为宏观各向同性,不存在晶态材料中常见的位错、晶界等缺陷,普遍具有超高的断裂韧度和优异的耐腐蚀性能以及独特的自修复能力等特点[4-5]。高熵合金(high-entropy alloys, HEAs)的概念由叶均蔚于2004年正式提出:由5种或5种以上元素按等原子比或近等原子比构成的固溶体合金,其中各主元的原子分数在5%~35%之间[6];它是基于“化学无序”发展的新材料,其突破了传统合金材料基元的限制,创新性地从熵的角度开发和研究合金材料,开创了金属材料全新的研究领域。与传统合金相比,高熵合金没有明显的溶剂与溶质之分,表现出高强度、高韧性、抗辐照及优异的力学性能与软磁性能等特点[7-8]。
非晶合金多表现为“单一主元”或“双主元”特性,而高熵合金主张的是“多主元”理念。两者之间貌似存在较大差别,而且各成体系。但事实上,两者之间既密切相关,又存在交叉互融。根据非晶形成的“混乱原理”和井上三原则,热力学的高熵效应和动力学的迟滞扩散效应,使得多组元高熵合金体系可能具有优良的非晶形成能力。在特定的制备条件下,部分高熵合金满足高结构熵和高混合熵时,会形成非晶结构,实现合金在具有紧密拓扑结构的同时还拥有高度的化学无序状态,进而形成强度与韧性相兼容的高熵非晶合金[9-11]。
迄今为止发现的所有高熵非晶合金体系主要分为两大类:一类是等原子比的纯金属组成体系,如具有良好生物相容性的CaMgZnSrYb[12]、高热稳定性的Ge-NbTaTiZr[13]、抗辐照性能突出的ZrTiHfCuBeNi[14]、拥有优异磁制冷性能的GdTbDyHoErYNiCoAgAl[15]以及在氢渗透与分离领域具有应用潜能的TiZrNiCoM(M=Cu,Hf)和NbNiZrTiN(N=Co, Cu)[16]等。另一类为等原子比或近等原子比的“过渡金属(Fe, Co, Ni等)—类金属(C, P, B, Si)”组成体系,如具有良好软磁性能的Fe25Co25Ni25(B0.6Si0.4)25与Fe25Co25Ni25(B0.7Si0.3)25[17]、高硬度高韧性的Fe20Co20Ni20Cr10Mo10B20[18]、高塑性与优异耐腐蚀性能的条带状Fe30Co25Ni25P14B6与Fe29Co26Ni25P14B6[19]等。同时高熵非晶合金还具有优异的力学性能[20-23],但受到苛刻的制备工艺和非晶形成能力的限制,大部分仅以毫米棒形式存在且材料成本较高,这严重制约了其工程推广应用。因此,突破高熵非晶合金难以规模化生产的技术“瓶颈”、降低制备成本,拓宽其应用领域是学者们的共同期望。
2 高熵非晶材料的制备方法及组织与性能高熵非晶合金的制备方法与高熵合金有一定的相似性,主要包括:熔铸法(melt casting process)、机械合金化法(mechanical alloying method, MA)、粉末冶金法(powder metallurgy)、水/气雾化法(water atomization & gas atomization)、激光熔覆技术(laser cladding)、热/冷/等离子喷涂法(thermal spraying, cold spraying & plasma spraying)、磁控溅射法(magnetron sputtering)、电子束蒸发沉积法(electron beam evaporation)、电化学沉积法(electrochemical deposition)、等离子体基离子注入法(plasma-based ion implantation, PBII)、放电等离子/真空热压烧结技术(spark plasma sintering, SPS & vacuum hot pressing sintering, VHPS)、自蔓延高温合成法(self-propagation high-temperature synthesis, SHS)等。具体采用何种制备工艺,主要依据高熵非晶材料的不同形态和用途来确定;在实际应用中为了获得成型性好、生产成本可控、性能优异的功能材料,通常将几种制备工艺结合使用。目前高熵非晶合金主要涵盖块体、涂层(薄膜)、粉体、箔带及高熵合金基复合材料等,不同形态的高熵非晶合金的制备方法及其特点如表 1所示[1-2, 10, 12-13, 18, 20, 24-42]。
| Form | Component | Preparation method | Characteristic | Reference | ||
| dc/mm or phase | Hardness | Performance characteristic | ||||
| Bulk | Gd20Tb20Dy20Al20Co(Ni)20 | Vacuum arc melting+ | 1 | Excellent soft magnetic | [24] | |
| Er20Dy20Co20Al20Gd20 | copper mold casting | 1.5 | and room-temperature | [25] | ||
| Er20Dy20Co20Al20Tm20 | 1 | magnetocaloric | [25] | |||
| Fe25Co25Ni25Mo5P10B10 | Fluxing treatment and J-quenching technique |
1.2 | properties |
[26] |
||
| Ti20Zr20Cu20Ni20Be20 | Copper mold injection casting method |
3 | High fracture strength & good GFA | [27] | ||
| Ti20Zr20Hf20Cu20Ni20 | 1.5 | Good mechanical properties | [10] | |||
| Pd20Pt20Cu20Ni20P20 | Fluxed water quenching | 10 | [2] | |||
| FeCrAlCuNiSi | MA+ultrahigh pressure consolidation |
1100- 1120HV |
Anti-corrosion & excellent mechanical properties | [28] | ||
| CuMnTiMoCrFe | Uniform droplets spraying micro-fabrication method | 1200HV | As a consumable for 3D printing |
[29] | ||
| Fe20Ni20Cr25Mo15(P0.6C0.2B0.2)20 | Induction-melting method | 1 | 1107HV | Exceptional thermal stability & corrosion resistance | [30] | |
| Ca20Mg20Zn20Sr20Yb20 | >2 | Bio-corrosion & biocompatibility |
[12] | |||
| Zn20Ca20Sr20Yb20(Li0.55 Mg0.45)20 |
3 | Good conductivity, polymerlike thermoplastic manufacturability & ultr-alow elastic moduli |
[1] | |||
| Ti16.7Zr16.7Hf16.7Cu16.7 Ni16.7Be16.7 |
A copper mould suction casting technique |
>15 | High fracture strength | [31] | ||
| Thin film/ |
(FeCoNi)25(AlTiZr)75 | DC/RF/reactive magnetron sputtering |
FCC+amorphous and nanocrystalline |
Anti-corrosion property, good tribological |
[32] | |
| coatings | (TiAlCrNbY)-C | FCC+amorphous | performance, excellent | [33] | ||
| (AlCrSiTiZr)77.6N22.4 | Amorphous | thermal stability & | [34] | |||
| TiAlCrSiV | superior tensile properties | [35] | ||||
| GexNbTaTiZr (x=0.5, 1) | [13] | |||||
| AlCrTaTiZr | [36] | |||||
| Al0.3CoCrFeNi with Ni-P amorphous film |
Electroless-plating method | [37] | ||||
| FeCrCoNiSiB | Laser cladding | FCC+amorphous | [38] | |||
| FeCoNiBSi | FCC+BCC+ amorphous |
400HV0.1 | [39] | |||
| Ribbon | Gd20Tb20Dy20Al20Fe20 | Arc melting+spinning Cu roller |
Amorphous | Large magnetocaloric effect |
[24] | |
| Zr20Ti20Hf20Cu20Co20 Zr20Ti20Hf20Cu20Fe20 |
Melt spinning+copper mold casting method |
Good mechanical properties |
[10] | |||
| (Fe, Co, Ni, Cr, Mo)-B | Melt spinning | [18] | ||||
| Al20Ce20La20Ni20Y20 | Arc melting+the melt-spun technique |
Potential-upsurge in the apparent activation energy of tensile creep | [20] | |||
| Powder | FeCoNiSi0.4Al0.4 | As-cast dry-milling process |
Nano-crystal and nano-amorphous |
Tuning magnetic properties |
[40] | |
| Zr2.5FeNiSi0.4B0.6 | MA method (milling 180 h) |
Full amorphous | High thermal stability | [41] | ||
| Metal matrix composites |
Al0.6CoCrFeNi HEA particle reinforced Al65Cu16.5Ti18.5 amorphous alloy composite |
Ball milling+SPS | (Amorphous/nano- crystalline)+ ID layer+(BCC+ FCC) |
Outstanding yield strength & low plasticity |
[42] | |
“真空电弧熔炼+铜模铸造”是制备块体高熵合金最常见的工艺[6]。Huo等[24]以Gd20Tb20Dy20Al20M20(M=Fe, Co, Ni)为原料,利用氩气保护在真空电弧熔炼炉中制备了一系列可作为磁热效应材料的块体高熵非晶态合金,最终样品通过X射线衍射仪(射线源为CuKα靶)呈现典型馒头峰的非晶态显微结构。Ding等[27]也采用相同方法获得了临界尺寸为3 mm的Ti20Zr20Cu20Ni20Be20高熵块体非晶合金,经实验测试发现其断裂强度为(2315±30) MPa,远超过传统钛基与镍基非晶合金,其玻璃化转变温度Tg=683 K,净化温度Tx=729 K。Cao等[43]在Zr20Cu20Hf20Ti20Ni20高熵块体非晶合金的基础上,通过研究发现微合金化氧元素会增加原子堆积密度,减少晶体相的成核和生长,进而提高合金的玻璃形成能力。类似地,Li等[25]也发现了具有自旋玻璃行为和优异磁热效应的新型Er20Dy20Co20Al20RE20(RE=Gd, Tb, Tm)高熵非晶块体合金,该材料可作为未来低温磁性制冷剂使用。Ding等[31]采用电弧熔炼的工艺获得了最大临界尺寸为15 mm的TiZrHfCuNiBe高熵非晶合金,经DSC曲线分析并估算其过冷液相区范围ΔTx=70 K,约化玻璃转变温度Trg=0.619。Inoue和叶均蔚课题组[2]采用熔体快淬法发现首个临界尺寸为厘米量级的Pd20Pt20Cu20Ni20P20高熵块体非晶合金,计算了该合金的过冷液相区范围ΔTx=65 K,约化玻璃转变温度Trg=0.71>0.69,说明其非晶态形成能力较强。Ma等[10]采用同样工艺并经铜模铸造的方法获得了直径为1.5 mm的棒状Ti20Zr20Hf20Cu20Ni20金属玻璃,其玻璃化转变温度Tg=658 K,约化玻璃转变温度Trg=0.57。Zhao等[44]通过真空感应熔炼铜模冷却的方法制备了临界尺寸为5 mm的Sr20Ca20Yb20Mg20Zn20与Sr20Ca20Yb20Mg20Zn10Cu10高熵非晶合金,其约化玻璃转变温度Trg分别为0.56与0.55。Li等[30]通过相同工艺制备了多个临界尺寸分别为1, 1.5 mm与2 mm的FeNiCrMo(P, C, B)块体高熵非晶合金,其中Fe30Ni20Cr25Mo5(P0.6C0.2B0.2)20的维氏硬度为974HV,屈服强度σf =3.3 GPa,冷液相区范围ΔTx=69 K,约化玻璃转变温度Trg=0.526。席生岐等[45]采用机械合金化非平衡制备工艺制得了一种具有高耐蚀性的六元非晶态高熵合金粉末,并经超高压固结与室温冷却后获得了硬度在1100~1120HV的非等原子比FeCrAlCuNiSi高熵非晶合金,经电化学测试发现其耐蚀性优于304L不锈钢。
2.2 涂层(薄膜)类高熵非晶合金激光熔覆技术制备的合金涂层与基材结合强度高,制得的涂层致密均匀。陈伟等[39]采用该方法制得了一种非晶含量最高可达64.3%(体积分数)的五元高熵非晶合金涂层,经观察测试发现:涂层表面平滑均匀且有光泽,显微维氏硬度在400HV0.1以上;着色探伤检测后涂层不存在裂纹,表面性能优异。Shu等[38]通过该工艺获得了FeCrCoNiSiB高熵非晶涂层,其中非晶/晶体层的非晶相占49%(体积分数),其余为FCC γ-(Fe, Ni)固溶体。涂覆层的磨损机制主要是磨粒磨损和氧化磨损,且非晶/晶体层磨损率比枝晶层磨损率低25.9%。磁控溅射技术具有沉积过程稳定,工艺参数易控制,薄膜的结合力好、不易掉落,可规模化和绿色环保等特点,广泛应用于涂层或薄膜的批量生产。Liu等[46]采用直流磁控溅射法在惰性混合气氛中制备了致密均匀的FeCoNiCuVZrAl高熵非晶薄膜,其硬度和杨氏模量最高可达12 GPa和166 GPa。Hsueh等[34]采用直流反应磁控溅射的方法在6061铝合金和低碳钢基材上制备了(AlCrSiTiZr)100-xNx高熵合金薄膜,研究了氮气流率和基底偏压对合金薄膜耐腐蚀性能的影响。在不施加基底偏压的条件下,氮气流率为30%时高熵合金薄膜具有最佳的耐蚀性能。当基底偏压为-100 V时,有效提高了(AlCrSiTiZr)N非晶薄膜的耐蚀性能,这是因为基底偏压引起薄膜致密化效应和薄膜存在压应力。Braic等[33]在Ar+CH4的气氛中,采用反应磁控溅射的方法成功制备了(TiAlCrNbY)C高熵合金碳化物薄膜,研究发现碳含量会影响薄膜的微观结构,不含碳的薄膜显示出完全的FCC固溶体结构;当碳含量为46%(原子分数,下同)和55%时,碳化物呈现NaCl型面心立方结构(FCC),并且具有(111)择优取向;当碳含量为69%和82%时,薄膜呈现非晶态结构。
2.3 其他类高熵非晶合金机械合金化法具有易形成亚稳相、组织成分均匀等优点,是一种较为成熟、简单有效的粉末制备技术。Shu等[47]采用机械合金化法制备了CoCrCuFeNiV0.6高熵合金,同时研究发现随着球磨时间的延长,合金成分均匀性增加,组织愈加细化;在球磨21 h后机械合金化程度趋于稳定,合金粉末显示有非晶相出现。放电等离子烧结技术具有烧结速率高,制备工艺周期短的特点。Tan等[42]利用放电等离子烧结技术制备了一种塑性变形能力不佳但屈服强度在(3120±80) MPa的高熵合金颗粒增强铝基非晶合金复合材料。Zhang等[40]采用干磨制粉法在不添加任何添加剂的前提下,将带状合金试样在行星球磨机中研磨60 h制备出具有双相纳米晶和纳米非晶微观结构的高熵合金粉末,同时研究发现延长退火时间会导致FCC固溶体相含量的增加。单辊快淬法多用于制备非晶或亚稳相微晶薄带等材料,具有冷却速率高的特点。Biljaković等[48]利用该技术制备了4种厚度为20 μm的(TiZrNbCu)1-xNix(x=0, 0.125, 0.15, 0.2,0.25)高熵非晶带状材料,并研究了其电子结构和性能特点。
3 高熵非晶合金的耐腐蚀性能高熵非晶合金结构简单、成分均匀,可消除材料内部元素贫富分化及不同区域电势差异,降低点蚀发生概率,延缓腐蚀速率,并且表面较高的化学反应活性促进钝化层的快速形成和修复;非晶合金在成分与结构上比传统合金更加均匀,同时非晶结构可进一步提高合金宏观强度与耐磨性。按照合金主元物理性质的不同进行划分,可将高熵非晶材料具体细分为:轻质高熵非晶合金、3d过渡族高熵非晶合金和难熔高熵非晶合金三大类,特别是前两类合金在高湿、高盐雾及海洋微生物等多物理场耦合的极端海洋环境中耐腐蚀能力更强;采用电化学分析方法测试金属涂层的抗腐蚀性具有短时高效、操作便捷的优点,更能深入客观地研究材料的腐蚀行为,表 2是部分高熵非晶合金材料与常见钢铁材料的电化学实验结果[28, 30, 32, 49-58]。由表 2可见,高熵非晶合金的自腐蚀电位比钢铁材料更正,发生腐蚀的倾向也更小;其自腐蚀电流密度比钢铁材料至少小2~3个数量级,说明其实际腐蚀程度更低;极化电阻越大,材料越不易发生腐蚀。
| Material | Corrosive environment | Ecorr/V | icorr/(A·cm-2) | Rp/(kΩ·cm-2) | Reference |
| VAlTiCrSi | Artificial sea water | -0.285 | 4.68×10-9 | [49] | |
| CuZrAlTiNiSi | Seawater solution | -1.140 | 6.21×10-4 | [50] | |
| Fe24Co24Ni24V6Si13B9 | 0.1 mol/L NaCl | -0.400 | 5.21×10-6 | [51] | |
| Fe24Co24Ni24Cr6Si13B9 | 0.1 mol/L NaCl | -0.270 | 1.25×10-6 | ||
| Fe24Co24Ni24Mo6Si13B9 | 0.1 mol/L NaCl | -0.490 | 3.70×10-5 | ||
| Fe24Co24Ni24Nb6Si13B9 | 0.1 mol/L NaCl | -0.380 | 4.89×10-6 | ||
| Fe20Ni20Cr25Mo15(P, C, B)20 | 0.5 mol/L H2SO4 | 1.98×10-4 | 2.30×10-9 | [30] | |
| 3%NaCl | 0.77×10-4 | 3.80×10-9 | |||
| 1 mol/L HCl | 2.26×10-4 | 7.30×10-9 | |||
| (Fe, Co, Ni, Cr)80B20 | 0.1 mol/L H2SO4 | -0.226 | 1.79×10-7 | [52] | |
| 0.1 mol/L HCl | -0.158 | 6.26×10-6 | |||
| 3.5%NaCl | -0.153 | 3.66×10-7 | |||
| FeSiBAlNiCo0.2 | 3.5%NaCl | -1.370 | 1.04×10-7 | [53] | |
| FeSiBAlNiCo0.8 | 3.5%NaCl | -0.860 | 4.79×10-7 | ||
| Fe3Cr2Al2CuNi4Si5 | 3.5%NaCl | -0.190 | 4.00×10-6 | [28] | |
| (FeCoNi)25(AlTiZr)75 | 3.5%NaCl | -0.260 | 6.20×10-8 | 422.00 | [32] |
| TiAlCrSiV | 3.5%NaCl | -0.541 | 6.14×10-6 | 11.36 | [54] |
| V10.4Nb10.5Mo10.5Ta11.2 W10.5Cr16.3B28.6 |
3.5%NaCl | 0.060 | 1.50×10-7 | 172.80 | [55] |
| SS 310 | 3.5%NaCl | -0.310 | 1.36×10-6 | 19.12 | |
| 45 steel | 3.5%NaCl | -0.712 | 3.72×10-6 | 7.62 | [56] |
| SS 304 | 3.5%NaCl | -0.282 | 1.69×10-7 | [57] | |
| SS 316L | 3.5%NaCl | -0.164 | 5.38×10-8 | ||
| 1Cr18Ni9Ti | 3.5%NaCl | -0.215 | 7.44×10-8 | ||
| Q235 steel | 3.5%NaCl | -0.570 | 7.10×10-4 | [58] | |
| T10 steel | Seawater solution | -1.050 | 6.39×10-4 | [50] |
高熵合金的高熵效应使其倾向于形成简单的固溶体结构,不易和金属间化合物产生原电池反应;独特的迟滞扩散效应能有效阻止氯离子侵入,增强微区耐蚀能力,并且含Co, Cr, Ni, Ti, Al, Mo等钝化元素能够促进钝化膜形成,使HEAs在高浓度的腐蚀溶液环境下均表现出优异的耐蚀性能。Al, Mg, Ti等金属元素及B, C, Si等类金属元素的密度都较小,且类金属元素普遍存在原子尺寸小、具有高共价键特性并促进钝化膜生成的特点。同时Al与Mg的电化学性能活泼,可对钢铁基体形成有效的阴极保护,特别是Al基非晶合金[59](如Al-Ni-Ti)可自发形成较为致密的Al2O3保护膜,能够降低腐蚀速率,具有优异的抗腐蚀性。
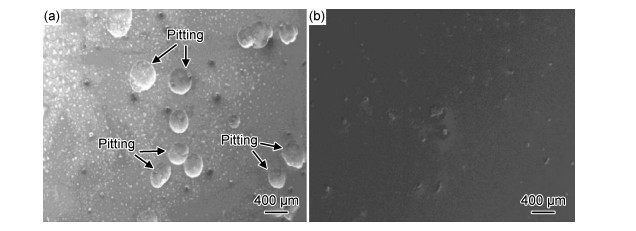
Zheng等[49]采用直流磁控溅射法成功制备了光滑致密且具有高硬度和耐腐蚀性特点的VAlTiCrSi高熵非晶合金薄膜(high-entropy alloy film, HEAF),如图 1所示。经电化学实验测得其自腐蚀电位为-0.285 V,腐蚀电流密度达到4.68×10-9A/cm2,较304不锈钢低一个数量级,说明该材料适用于海水腐蚀性环境下的基体防护。Wang等[60]制备了(Fe-Co-Ni)x(Al-Ti-Zr)100-x(x=15, 20, 25)高熵非晶合金涂层,研究发现3种涂层均由FCC纳米晶固溶体相和非晶相组成,其晶粒尺寸从25 nm (x=15)增加到40 nm (x=20)和52 nm (x=25);Fe-Co-Ni含量的增加有利于晶体的聚集与生长,从而降低了晶间腐蚀速率;且在三者中(Fe-Co-Ni)25(Al-Ti-Zr)75的自腐蚀电位最正(Ecorr=-260 mV),自腐蚀电流密度最低(icorr=0.062 μA/cm2),极化电阻最大(Rp=4.22×105Ω)。Han等[50]采用机械合金化和真空热压烧结技术,在T10钢基体上制备了成分分布均匀且含有非晶相的CuZrAlTiNiSi高熵合金涂层(high-entropy alloy coating, HEAc)。研究发现铝原子从涂层扩散到基体,使HEAc与基体的结合更加紧密且表面无气孔及缺陷。由于第二相强化和固溶强化的作用,涂层的平均显微硬度达到927HV0.2,是基体的3倍以上;经电化学测试HEAc的钝化区域更宽(ΔE=0.83 V),因而在海水环境下具有更佳的抗点蚀能力。Zhang等[61]采用磁控溅射技术制备出含有非晶相的AlCrMoNbZr和FCC相的(AlCrMoNbZr)N多层镀层,通过30天在360 ℃和18.7 MPa条件下的静态腐蚀实验表明,AlCrMoNbZr/(AlCrMoNbZr) N多层结构可以有效阻止Al原子的迁移与γ-AlOOH的形成,从而具有优异的耐腐蚀性能。
3d过渡族金属基合金的强度较其他合金更为突出,面向海洋环境应用的Fe基[62]和Co基[63]块体非晶的压缩断裂强度分别达到4800 MPa和5300 MPa,且耐腐蚀性比常规不锈钢高10000倍。3d过渡族金属元素作为主体的高熵合金,原子尺寸差异小,负混合焓小、电负性相近,容易形成单相固溶体;其中Cr, Ni可自发形成钝化膜,使合金具有优异的本征耐腐蚀性能[64]。此外,通过添加高共价键特性或促进钝化膜生成的类金属元素(B, Si, P等),不仅能够提高高熵非晶合金的强度,还可以形成一定负混合焓,促进高熵非晶结构的形成,提高材料的耐蚀能力。
3.2.1 元素含量对耐腐蚀性能的影响Fe, Co, Ni等是3d过渡族金属基高熵合金常用的元素,特别是FeCoNiCrMn高熵合金具有单相面心立方结构,超低温强度和韧性尤为优异。Luo等[65]研究了该HEAs与304不锈钢室温条件下在0.1 mol/L硫酸溶液中浸泡30天后的腐蚀行为。实验结果发现高熵合金的钝化膜主要成分为Cr, Fe和Ni的氢氧化物与Mn, Co的氧化物;304不锈钢的钝化膜主要成分为Fe和Cr的氧化物。在HEAs的钝化膜中存在贫Cr区和富Fe区,且其厚度大于304不锈钢上的钝化膜厚度;氢氧化物的形成与贫Cr区的出现是HEAs耐腐蚀性能下降的主要原因。
Ding等[52]同时研究了Fe100-xBx(x=18~24)非晶合金和(Fe, Co, Cr, Ni)100-xBx(x=18~26)高熵非晶合金的微观结构与性能特征,发现x=20%时HE-MGs的维氏硬度最高可达1130HV;通过在3.5%NaCl溶液中进行电化学测试得出HE-MGs的自腐蚀电位为-153.3 mV,腐蚀电流密度为0.366 μA/cm2,远优于Fe-B非晶合金,说明非晶相的形成大幅度提高了合金的耐腐蚀性。Wang等[53]采用机械合金化工艺制备了FeSiBAlNiCox(x=0.2, 0.8)合金粉末,并采用SPS技术对粉末进行140 h的烧结。研究发现粉末和烧结试样的微观结构均呈现完全非晶结构。Co含量的增加明显提高了高熵非晶粉体的玻璃形成能力和热稳定性,进而缩短了完全非晶相形成的时间,提高了晶化开始温度。FeSiBAlNiCo0.2的击破电位更高,腐蚀电流密度更低且钝化区间更宽,因而具有更好的耐腐蚀性。Li等[30]研究发现FeNiCrMo(P, C, B)高熵非晶合金具有突出的力学性能;同时由于高熵效应,合金的加热结晶过程更缓慢。由于富Cr区的存在以及大原子半径元素Mo可延缓腐蚀速率并与类金属元素间形成较大的负混合热焓,在与周围临近原子形成类似网状结构或骨架结构的同时,降低了原子的有序化程度,增强过冷熔体的稳定性并抑制晶体相的形核和长大,有利于非晶相的形成,所以该类材料在酸性溶液和NaCl溶液中表现出优异的耐腐蚀性。
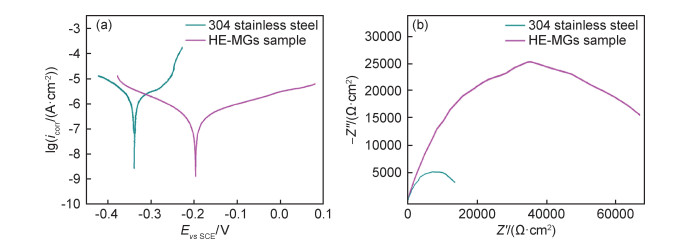
3.2.2 添加易钝化元素对耐腐蚀性能的影响合金成分是影响3d过渡族金属基高熵非晶材料强度和耐蚀性的重要因素,在合金中添加适量易致钝的强耐蚀元素Cr, Al, Zr, Nb, Ni等以及在淡水和海水具有耐蚀性的Cu元素,能够从整体上提高合金的耐腐蚀能力。Yang等[28]通过机械合金化与超高压固结成形工艺制备了一种硬度为1150HV的新型六元非等原子比Fe3Cr2Al2CuNi4Si5高熵非晶合金。图 2显示的是该合金经电化学测试的动电位极化曲线与Nyquist曲线,由图 2可知,高熵非晶合金的自腐蚀电位为-0.19 V,腐蚀电流密度为4×10-6A/cm2,结果证实Ni, Cr, Al等元素和相对均匀的微观结构有利于钝化膜的形成,从而可以提升合金的耐蚀能力。余红雅等[51]在具有完全非晶结构的Fe25Co25Ni25(B, Si)25高熵合金基础上,采用真空电弧熔炼与单辊甩带相结合的手段,制备了一系列具有优异软磁性能和热稳定性良好的Fe24Co24Ni24X6Si13B9(X为Cr, Mo, Nb或V)高熵非晶合金。通过对其在0.1 mol/L NaCl溶液中进行耐腐蚀测试,发现该种新型材料在高温环境下具有抗氧化腐蚀的特点且仍旧保持非晶软磁性能,可应用于航空发动机与高温传感器等设备。
制备工艺参数对高熵非晶材料的纯度、成分均匀性和宏观性能均有一定影响。Shu等[66]采用不同激光功率在H13钢表面制备了CoCrBFeNiSi高熵合金涂层,具体包括枝晶状的底层、过渡层和上部的非晶层。涂层中的非晶含量取决于激光功率密度;非晶含量越多,涂层的显微硬度越高,对HCl和NaCl溶液的耐腐蚀性越好。反之,非晶含量降低时,涂层表面出现较严重的黏着磨损和氧化磨损,使其耐腐蚀性与耐磨损性均下降。
3.3 难熔高熵非晶合金的耐腐蚀性能由于组成高熵非晶合金的元素众多,每一种元素均有其独特的物理化学性质,不同元素之间的相互作用可以使高熵非晶合金表现出复合效应;该效应更加强调元素的某些性质最终通过影响合金的宏观性能得以呈现,因而印度学者Ranganathan将其命名为“鸡尾酒”效应[67]。随着航空航天、石油化工、能源冶金等现代工业的迅猛发展,对材料的使用环境愈加严苛,提高先进设备热端部件的抗高温氧化腐蚀与抗高温熔盐腐蚀性能并延长其服役寿命势在必行。近年来,许多学者聚焦于材料的高温腐蚀行为与防护研究,如:激光选区熔化制备的Inconel 718镍基高温合金[68]、磁控溅射法制备的FeCrAlY薄膜[69]以及热障涂层新材料Mg2SiO4等[70]。
以难熔金属(Nb, Ta, Mo, W, Zr, Ti等)为主元形成的高熵合金具有极高的高温组织稳定性和耐高温氧化等超高温理化性能[71]。通过具体分析合金组元的元素性质能够发现,Nb能够显著提升合金的硬度及蠕变强度,在合金中添加Ta有助于提高其比强度和耐高温腐蚀性能,同时Nb与Ta均可起到固溶强化的作用;Mo能细化晶粒并使合金的力学性能提高,W不但可以提高合金的高温强度而且能显著提升耐磨性和切削性,也常被作为Nb合金的强化元素,进一步提升其高温蠕变性能;Zr的作用与Nb, Ta相似,加入适量Zr能够净化和细化晶粒并改善金属材料的冲压性能[72-73]。此外,在合金体系中加入Al, Ti, Cr等能够氧化生成致密氧化层薄膜的保护性元素,可以提高合金材料的耐腐蚀性能;掺杂类金属元素形成的难熔金属基高熵合金,其高温力学性能可得到进一步提高。通过将难熔高熵合金非晶化,消除了材料内部元素贫富分化和不同区域电势差异,增强耐蚀能力,使高熵非晶合金的强度提升。此外,在高熵合金制备过程中采用快速淬火工艺可以延缓合金元素的扩散,避免出现偏析与成分起伏,获得更加均匀的微观结构与成分分布,进而增强其抗高温腐蚀性能。
Hung等[55]采用磁控溅射工艺制备了VNbMoTaW高熵合金,之后加入易致钝元素Cr和具有提高硬度的B元素后,原有体心立方(BCC)结构的HEA涂层转变为具有非晶结构的VNbMoTaWCrB高熵合金涂层,进一步提高其高温抗氧化与耐腐蚀性能。实验发现V10.4Nb10.5Mo10.5Ta11.2W10.5Cr16.3B28.6难熔高熵非晶合金涂层具有优异的耐腐蚀性,经相关测试其极化电阻Rp=172.8 kΩ·cm-2,最高硬度为(18.4±0.5)GPa;在1 h的500 ℃氧化实验后涂层具有良好的热稳定性,硬度为(15.9±1.1) GPa。
4 结束语高熵非晶合金的出现拓宽了工程材料使用的可选择范围,具有很大的应用潜能和广阔的发展前景[74-76];特别是作为一种面向海洋极端环境服役的新型材料,未来可从以下方面进行深入研究及推广使用:
(1) 对于高熵合金的设计,已逐渐从“鸡尾酒”式的调配方法转变为通过材料基因工程理念实现合金组元的快速筛选,并采用高通量计算与设计实现系列样品的平行合成[77]。通过材料模拟计算技术,在短期内可对新型材料进行全方位性能表征,并能够从微观与介观尺度上阐明微纳结构与其力学及耐蚀等综合性能的内在关联。而对于高熵非晶材料,仍存在许多未知的合金组分有待探索,其材料基因工程建设亟待完善。目前仅有少数学者对该材料的成相规律与拓扑参数进行研究,Hu等[78]通过利用真空电弧熔炼技术制备了大量表面平滑光整且具有良好韧性的TiZrHfM, TiZrHfMM, TiZrHfMMM (M=Fe,Cr,V,Nb,Al,Ag,Cu,Ni)高熵合金薄带,并提出了一种采用原子半径修正值计算出的拓扑参数来区分晶态和非晶态合金。进一步发现由于HCP和FCC元素之间的混合焓比HCP和BCC元素之间的混合焓更负,所以HCP和FCC元素组合成的高熵合金易于形成非晶。
近年来,材料研发主要由“炒菜”模式逐渐转变为以数据驱动为特点的新范式,而以“数据密集型科学发现”为主要特征的机器学习已经广泛应用于新材料的设计和研发[79-81],相信未来会有更多高熵非晶材料体系不断涌现;同时试图建立合金成分-微观结构-宏观性能-成分优选的评价反馈体系,搭建计算模拟与实验验证的基本映射关联架构,从更深角度揭示高熵非晶合金耐蚀性能的结构起源也将会成为其研究重点之一。
(2) 目前针对高熵非晶涂层的耐蚀性能仅停留在宏观电化学腐蚀实验及腐蚀后的微观表征方面,而对于其微观尺度耐蚀机理方面的研究还有待深化,微观测试与表征手段的精度仍有改善空间;同时,面向工程应用的实况下腐蚀实验实施得较少,对于工况环境下的腐蚀失效机制及耐腐蚀机理尚需完善。
(3) 高熵非晶粉体的组织结构、形貌和热稳定性影响涂层结构与物化性能等。探究不同工艺条件对粉末物理参数及内部组织特性的影响规律,完善粉末制备的最佳工艺,为实现高效批量制备高品质粉末提供材料支持将会成为高熵非晶材料推广与应用的关键。
因此,开发具有耐腐蚀性的高熵非晶材料,对解决未来前瞻性、战略性领域中大型装备材料面临的腐蚀和老化等严重失效问题,实现极端海洋环境服役条件下大型装备的长效防护具有重大而深远的意义。
| [1] |
ZHAO K, XIA X X, BAI H Y, et al. Room temperature homogeneous flow in a bulk metallic glass with low glass transition temperature[J]. Applied Physics Letters, 2011, 98(14): 141913. DOI:10.1063/1.3575562 |
| [2] |
TAKEUCHI A, CHEN N, WADA T, et al. Pd20Pt20Cu20Ni20P20 high-entropy alloy as a bulk metallic glass in the centimeter[J]. Intermetallics, 2011, 19(10): 1546-1554. DOI:10.1016/j.intermet.2011.05.030 |
| [3] |
KLEMENT W, WILLENS R H, DUWEZ P. Non-crystalline structure in solidified gold-silicon alloys[J]. Nature, 1960, 187(4740): 869-870. |
| [4] |
INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys[J]. Acta Materialia, 2000, 48(1): 279-306. DOI:10.1016/S1359-6454(99)00300-6 |
| [5] |
汪卫华. 非晶态物质的本质和特性[J]. 物理学进展, 2013, 33(5): 177-351. WANG W H. The nature and properties of amorphous matter[J]. Progress in Physics, 2013, 33(5): 177-351. |
| [6] |
YEH J W, CHEN S K, LIN S J, et al. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes[J]. Advanced Engineering Materials, 2004, 6(5): 299-303. DOI:10.1002/adem.200300567 |
| [7] |
MIRACLE D B, SENKOV O N. A critical review of high entropy alloys (HEAs) and related concepts[J]. Acta Materialia, 2017, 122: 448-511. DOI:10.1016/j.actamat.2016.08.081 |
| [8] |
LI Z Z, ZHAO S T, RITCHIE R O, et al. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys[J]. Progress in Materials Science, 2019, 102: 296-345. DOI:10.1016/j.pmatsci.2018.12.003 |
| [9] |
LI R X, ZHANG Y. Entropy and glass formation[J]. Acta Physica Sinica, 2017, 66(17): 350-357. |
| [10] |
MA L Q, WANG L M, ZHANG T, et al. Bulk glass formation of Ti-Zr-Hf-Cu-M (M=Fe, Co, Ni) alloys[J]. Materials Transactions, 2002, 43(2): 277-280. DOI:10.2320/matertrans.43.277 |
| [11] |
XING Q W, ZHANG Y. Amorphous phase formation rules in high-entropy alloys[J]. Chinese Physics B, 2017, 26(1): 65-73. |
| [12] |
LI H F, XIE X H, ZHAO K, et al. In vitro and in vivo studies on biodegradable CaMgZnSrYb high-entropy bulk metallic glass[J]. Acta Biomaterialia, 2013, 9(10): 8561-8573. DOI:10.1016/j.actbio.2013.01.029 |
| [13] |
CHENG C Y, YEH J W. High thermal stability of the amorphous structure of GexNbTaTiZr (x=0.5, 1) high-entropy alloys[J]. Materials Letters, 2016, 181: 223-226. DOI:10.1016/j.matlet.2016.06.040 |
| [14] |
WANG Y, ZHANG K, FENG Y H, et al. Excellent irradiation tolerance and mechanical behaviors in high-entropy metallic glasses[J]. Journal of Nuclear Materials, 2019, 527: 151785. DOI:10.1016/j.jnucmat.2019.151785 |
| [15] |
HUO J T, WANG J Q, WANG W H. Denary high entropy metallic glass with large magnetocaloric effect[J]. Journal of Alloys and Compounds, 2019, 776: 202-206. DOI:10.1016/j.jallcom.2018.10.328 |
| [16] |
姚可夫, 丁红瑜.高熵非晶合金材料及其制备方法: ZL201310224674.5[P]. 2013-10-02. YAO K F, DING H Y. High entropy amorphous alloy material and its preparation method: ZL201310224674.5[P]. 2013-10-02. |
| [17] |
QI T L, LI Y H, TAKEUCHI A, et al. Soft magnetic Fe25Co25Ni25(B, Si)25 high entropy bulk metallic glasses[J]. Intermetallics, 2015, 66: 8-12. DOI:10.1016/j.intermet.2015.06.015 |
| [18] |
WANG F, INOUE A, KONG F L, et al. Formation, thermal stability and mechanical properties of high entropy (Fe, Co, Ni, Cr, Mo)-B amorphous alloys[J]. Journal of Alloys and Compounds, 2018, 732: 637-645. DOI:10.1016/j.jallcom.2017.10.227 |
| [19] |
董晓源, 许晓飞, 孙欢, 等.一种具有高塑性的铁基软磁高熵非晶合金及其制备方法和应用: ZL201810122747.2[P]. 2018-08-03. DONG X Y, XU X F, SUN H, et al. The invention relates to an Fe-based soft magnetic high entropy amorphous alloy with high plasticity and its preparation and application: ZL201810122747.2[P]. 2018-08-03. |
| [20] |
WU J L, ZHOU Z Y, YANG H, et al. Structure related potential-upsurge during tensile creep of high entropy Al20Ce20La20Ni20Y20 metallic glass[J]. Journal of Alloys and Compounds, 2020, 827: 154298. DOI:10.1016/j.jallcom.2020.154298 |
| [21] |
ZHAO S F, WANG H B, XIAO L, et al. High strain rate sensitivity of hardness in quinary Ti-Zr-Hf-Cu-Ni high entropy metallic glass thin films[J]. Physica E, 2017, 94: 100-105. DOI:10.1016/j.physe.2017.07.021 |
| [22] |
ZHAO Y Y, YE Y X, LIU C Z, et al. Tribological behavior of an amorphous Zr20Ti20Cu20Ni20Be20 high-entropy alloy studied using a nanoscratch technique[J]. Intermetallics, 2019, 113: 106561. DOI:10.1016/j.intermet.2019.106561 |
| [23] |
ZHOU Q, DU Y, HAN W C, et al. Identifying the origin of strain rate sensitivity in a high entropy bulk metallic glass[J]. Scripta Materialia, 2019, 164: 121-125. DOI:10.1016/j.scriptamat.2019.02.002 |
| [24] |
HUO J T, HUO L S, MEN H, et al. The magnetocaloric effect of Gd-Tb-Dy-Al-M (M=Fe, Co and Ni) high-entropy bulk metallic glasses[J]. Intermetallics, 2015, 58: 31-35. DOI:10.1016/j.intermet.2014.11.004 |
| [25] |
LI J, XUE L, YANG W M, et al. Distinct spin glass behavior and excellent magnetocaloric effect in Er20Dy20Co20 Al20RE20 (RE=Gd, Tb and Tm) high-entropy bulk metallic glasses[J]. Intermetallics, 2018, 96: 90-93. DOI:10.1016/j.intermet.2018.03.002 |
| [26] |
WU K N, LIU C, LI Q, et al. Magnetocaloric effect of Fe25Co25Ni25Mo5P10B10 high-entropy bulk metallic glass[J]. Journal of Magnetism and Magnetic Materials, 2019, 489: 165404. DOI:10.1016/j.jmmm.2019.165404 |
| [27] |
DING H Y, YAO K F. High entropy Ti20Zr20Cu20Ni20Be20 bulk metallic glass[J]. Journal of Non-Crystalline Solids, 2013, 364: 9-12. DOI:10.1016/j.jnoncrysol.2013.01.022 |
| [28] |
YANG X G, ZHOU Y, ZHU R H, et al. A novel, amorphous, non-equiatomic FeCrAlCuNiSi high-entropy alloy with exceptional corrosion resistance and mechanical properties[J]. Acta Metallurgica Sinica(English letters), 2020, 33: 1057-1063. DOI:10.1007/s40195-019-00977-1 |
| [29] |
徐轶, 陈亚.一种用于3D打印的高熵合金非晶粉末及其制备方法: ZL201610090111.5[P]. 2016-04-27. XU Y, CHEN Y. A kind of high entropy alloy amorphous powder for 3D printing and its preparation method: ZL201610090111.5[P]. 2016-04-27. |
| [30] |
LI Y, WANG S, WANG X, et al. New FeNiCrMo(P, C, B) high-entropy bulk metallic glasses with unusual thermal stability and corrosion resistance[J]. Journal of Materials Science & Technology, 2020, 42: 32-39. |
| [31] |
DING H Y, SHAO Y, GONG P, et al. A senary TiZrHfCuNiBe high entropy bulk metallic glass with large glass-forming ability[J]. Materials Letters, 2014, 125: 151-153. DOI:10.1016/j.matlet.2014.03.185 |
| [32] |
LIU J N, XING Z G, WANG H D, et al. Microstructure and fatigue damage mechanism of FeCoNiAlTiZr high-entropy alloy film by nanoscale dynamic mechanical analysis[J]. Vacuum, 2019, 159: 516-523. DOI:10.1016/j.vacuum.2018.10.061 |
| [33] |
BRAIC M, BRAIC V, BALACEANU M, et al. Characteristics of (TiAlCrNbY)C films deposited by reactive magnetron sputtering[J]. Surface and Coatings Technology, 2010, 204(12/13): 2010-2014. |
| [34] |
HSUEH H T, SHEN W J, TSAI M H, et al. Effect of nitrogen content and substrate bias on mechanical and corrosion properties of high-entropy films (AlCrSiTiZr)100-xNx[J]. Surface and Coatings Technology, 2012, 206(19/20): 4106-4112. |
| [35] |
LIN C H, DUH J G, YEH J W. Multi-component nitride coatings derived from Ti-Al-Cr-Si-V target in RF magnetron sputter[J]. Surface and Coatings Technology, 2007, 201(14): 6304-6308. DOI:10.1016/j.surfcoat.2006.11.041 |
| [36] |
LAI C H, LIN S J, YEH J W, et al. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings[J]. Surface and Coatings Technology, 2006, 201(6): 3275-3280. DOI:10.1016/j.surfcoat.2006.06.048 |
| [37] |
XIA Z H, ZHANG M, ZHANG Y, et al. Effects of Ni-P amorphous films on mechanical and corrosion properties of Al0.3CoCrFeNi high-entropy alloys[J]. Intermetallics, 2018, 94: 65-72. DOI:10.1016/j.intermet.2017.12.021 |
| [38] |
SHU F Y, LIU S, ZHAO H Y, et al. Structure and high-temperature property of amorphous composite coating synthesized by laser cladding FeCrCoNiSiB high-entropy alloy powder[J]. Journal of Alloys and Compounds, 2018, 731: 662-666. DOI:10.1016/j.jallcom.2017.08.248 |
| [39] |
陈伟, 孙博, 韩剑, 等.高熵非晶合金涂层及其制备方法: CN201811653039.8[P]. 2019-03-08. CHEN W, SUN B, HAN J, et al. Preparation with amorphous and high-entropy coating: ZL201811653039.8[P]. 2019-03-08. |
| [40] |
ZHANG B, DUAN Y P, YANG X, et al. Tuning magnetic properties based on FeCoNiSi0.4 with dual-phase nano-crystal and nano-amorphous microstructure[J]. Intermetallics, 2020, 117: 106678. DOI:10.1016/j.intermet.2019.106678 |
| [41] |
SANG L M, XU Y. Amorphous behavior of ZrxFeNiSi0.4B0.6 high entropy alloys synthesized by mechanical alloying[J]. Journal of Non-Crystalline Solids, 2020, 530: 119854. DOI:10.1016/j.jnoncrysol.2019.119854 |
| [42] |
TAN Z, WANG L, XUE Y, et al. High-entropy alloy particle reinforced Al-based amorphous alloy composite with ultrahigh strength prepared by spark plasma sintering[J]. Materials & Design, 2016, 109: 219-226. |
| [43] |
CAO D, WU Y, LI H X, et al. Beneficial effects of oxygen addition on glass formation in a high-entropy bulk metallic glass[J]. Intermetallics, 2018, 99: 44-50. DOI:10.1016/j.intermet.2018.05.007 |
| [44] |
ZHAO K, JIAO W, MA J, et al. Formation and properties of strontium-based bulk metallic glasses with ultralow glass transition temperature[J]. Journal of Materials Research, 2012, 27(20): 2593-2600. DOI:10.1557/jmr.2012.214 |
| [45] |
席生岐, 杨喜岗, 周赟, 等.一种高耐蚀的非晶高熵合金及其制备方法: CN201611066271.2[P]. 2017-05-31. XI S Q, YANG X G, ZHOU Y, et al. The invention related to an amorphous high entropy alloy with high corrosion resistance and a preparation method: CN201611066271.2[P]. 2017-05-31. |
| [46] |
LIU L, ZHU J B, HOU C, et al. Dense and smooth amorphous films of multicomponent FeCoNiCuVZrAl high-entropy alloy deposited by direct current magnetron sputtering[J]. Materials & Design, 2013, 46: 675-679. |
| [47] |
SHU C Q, CHEN K, YANG H M, et al. Effect of V and ball milling time on microstructure and thermal properties of CoCrCuFeNiVx by mechanical alloying[J]. Physica B, 2019, 571: 235-242. DOI:10.1016/j.physb.2019.07.028 |
| [48] |
BILJAKOVIC' K, REMENYI G, FIGUEROA I A, et al. Electronic structure and properties of (TiZrNbCu)1-xNix high entropy amorphous alloys[J]. Journal of Alloys and Compounds, 2017, 695: 2661-2668. DOI:10.1016/j.jallcom.2016.11.179 |
| [49] |
ZHENG S, CAI Z, PU J, et al. A feasible method for the fabrication of VAlTiCrSi amorphous high entropy alloy film with outstanding anti-corrosion property[J]. Applied Surface Science, 2019, 483: 870-874. DOI:10.1016/j.apsusc.2019.03.338 |
| [50] |
HAN Z H, WANG D Z, CHEN X H, et al. Characterization and properties of CuZrAITiNiSi high entropy alloy coating obtained by mechanical alloying and vacuum hot-pressing sintering[J]. JOM, 2020, 72(3): 1254-1263. DOI:10.1007/s11837-020-04005-x |
| [51] |
余红雅, 左建亮, 刘仲武, 等.一种耐高温耐腐蚀高熵非晶软磁合金及其制备方法: ZL201910180210.6[P]. 2019-06-25. YU H Y, ZUO J L, LIU Z W, et al. The invention relates to a high entropy amorphous alloys with soft magnetic, anti-high temperature and anti-corrosion and its preparation method: ZL201910180210.6[P]. 2019-06-25. |
| [52] |
DING J, INOUE A, HAN Y, et al. High entropy effect on structure and properties of (Fe, Co, Ni, Cr)-B amorphous alloys[J]. Journal of Alloys and Compounds, 2017, 696: 345-352. DOI:10.1016/j.jallcom.2016.11.223 |
| [53] |
WANG W, LI B Y, ZHAI S C, et al. Alloying behavior and properties of FeSiBAlNiCox high entropy alloys fabricated by mechanical alloying and spark plasma sintering[J]. Metals and Materials International, 2018, 24: 1112-1119. DOI:10.1007/s12540-018-0047-1 |
| [54] |
LIN C H, DUH J G. Corrosion behavior of (Ti-Al-Cr-Si-V)xNy coatings on mild steels derived from RF magnetron sputtering[J]. Surface and Coatings Technology, 2008, 203(5/7): 558-561. |
| [55] |
HUNG S B, WANG C J, CHEN Y Y, et al. Thermal and corrosion properties of V-Nb-Mo-Ta-W and V-Nb-Mo-Ta-W-Cr-B high entropy alloy coatings[J]. Surface and Coatings Technology, 2019, 375: 802-809. DOI:10.1016/j.surfcoat.2019.07.079 |
| [56] |
梁秀兵, 范建文, 张志彬, 等. 铝基非晶纳米晶复合涂层显微组织与腐蚀性能研究[J]. 金属学报, 2018, 54(8): 1193-1203. LIANG X B, FAN J W, ZHANG Z B, et al. Microstructure and corrosion properties of aluminum base amorphous and nanocrystalline composite coating[J]. Acta Metallurgica Sinica, 2018, 54(8): 1193-1203. |
| [57] |
孙京丽, 邹丹, 金晶, 等. 三种常用不锈钢的耐局部腐蚀性能[J]. 材料研究学报, 2017, 31(9): 665-671. SUN J L, ZOU D, JIN J, et al. Localized corrosion resistance of three commonly-used stainless steels[J]. Chinese Journal of Materials Research, 2017, 31(9): 665-671. |
| [58] |
QIU X W. Corrosion behavior of Al2CrFeCoxCuNiTi high-entropy alloy coating in alkaline solution and salt solution[J]. Results in Physics, 2019, 12(3): 1737-1741. |
| [59] |
CHENG J B, FENG Y, YAN C, et al. Development and characterization of Al-based amorphous coating[J]. JOM, 2020, 72(2): 745-753. DOI:10.1007/s11837-019-03966-y |
| [60] |
WANG H D, LIU J N, XING Z G, et al. Microstructure and corrosion behaviour of AlCoFeNiTiZr high-entropy alloy films[J]. Surface Engineering, 2019, 36(1): 78-85. |
| [61] |
ZHANG W, TANG R, YANG Z B, et al. Preparation, structure, and properties of high-entropy alloy multilayer coatings for nuclear fuel cladding: a case study of AlCrMoNbZr/(AlCrMo-NbZr)N[J]. Journal of Nuclear Materials, 2018, 512: 15-24. DOI:10.1016/j.jnucmat.2018.10.001 |
| [62] |
HUANG B, ZHANG C, ZHANG G, et al. Wear and corrosion resistant performance of thermal-sprayed Fe-based amorphous coatings: a review[J]. Surface and Coatings Technology, 2019, 377(15): 124896. |
| [63] |
ZHANG G Y, ZHANG H, YUE S Q, et al. Preparation of non-magnetic and ductile Co-based bulk metallic glasses with high GFA and hardness[J]. Intermetallics, 2019, 107: 47-52. DOI:10.1016/j.intermet.2019.01.012 |
| [64] |
XU T, PANG S J, ZHANG T. Glass formation, corrosion behavior, and mechanical properties of novel Cr-rich Cr-Fe-Mo-C-B-Y bulk metallic glasses[J]. Journal of Alloys and Compounds, 2015, 625: 318-322. DOI:10.1016/j.jallcom.2014.09.166 |
| [65] |
LUO H, LI Z, MINGERS A M, et al. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution[J]. Corrosion Science, 2018, 134: 131-139. DOI:10.1016/j.corsci.2018.02.031 |
| [66] |
SHU F Y, ZHANG B L, LIU T, et al. Effects of laser power on microstructure and properties of laser cladded CoCrBFeNiSi high-entropy alloy amorphous coatings[J]. Surface and Coatings Technology, 2019, 358: 667-675. DOI:10.1016/j.surfcoat.2018.10.086 |
| [67] |
RANGANATHAN S. Alloyed pleasures: multimetallic cocktails[J]. Current Science, 2003, 85(10): 1404-1406. |
| [68] |
滕庆, 李帅, 薛鹏举, 等. 激光选区熔化Inconel 718合金高温腐蚀性能[J]. 中国有色金属学报, 2019, 29(7): 1417-1426. TENG Q, LI S, XUE P J, et al. High-temperature corrosion resistance of Inconel 718 fabricated by selective laser melting[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(7): 1417-1426. |
| [69] |
CUI Y, LI C J, LI J, et al. Characterization of FeCrAlY thin film deposited by magnetron sputtering and its corrosion resistance under high-temperature water vapor environment[J]. Surface Technology, 2020, 49(1): 72-78. |
| [70] |
陈思, 周鑫, 张豪, 等. 熔盐环境下热障涂层新材料Mg2SiO4的高温腐蚀研究[J]. 人工晶体学报, 2019, 48(8): 1534-1538. CHEN S, ZHOU X, ZHANG H, et al. High-temperature corrosion behavior of novel material Mg2SiO4 for thermal barrier coatings in molten salt environment[J]. Journal of Synthetic Crystals, 2019, 48(8): 1534-1538. |
| [71] |
ZHOU Q Y, SHEIKH S, OU P, et al. Corrosion behavior of Hf0.5Nb0.5Ta0.5Ti1.5Zr refractory high-entropy in aqueous chloride solutions[J]. Electrochemistry Communications, 2019, 98: 63-68. DOI:10.1016/j.elecom.2018.11.009 |
| [72] |
ZHANG M N, ZHOU X L, YU X N, et al. Synthesis and characterization of refractory TiZrNbWMo high-entropy alloy coating by laser cladding[J]. Surface and Coatings Technology, 2017, 311: 321-329. DOI:10.1016/j.surfcoat.2017.01.012 |
| [73] |
GUO Y X, LIU Q B. MoFeCrTiWAlNb refractory high-entropy alloy coating fabricated by rectangular-spot laser cladding[J]. Intermetallics, 2018, 102: 78-87. DOI:10.1016/j.intermet.2018.09.005 |
| [74] |
ZHANG M, GONG P, LI N, et al. Oxidation behavior of a Ti16.7Cu16.7Ni16.7Be16.7 high-entropy bulk metallic glass[J]. Materials Letters, 2019, 236: 135-138. DOI:10.1016/j.matlet.2018.10.056 |
| [75] |
ZHANG C, SONG A N, YUAN Y, et al. Study on the hydrogen storage properties of a TiZrNbTa high entropy alloy[J]. International Journal of Hydrogen Energy, 2020, 45(8): 5367-5374. DOI:10.1016/j.ijhydene.2019.05.214 |
| [76] |
DING Q Q, ZHANG Y, CHEN X, et al. Tuning element distribution, structure and properties by composition in high-entropy alloys[J]. Nature, 2019, 574(7777): 223-227. DOI:10.1038/s41586-019-1617-1 |
| [77] |
XU Y Q, BU Y Q, LIU J B, et al. In-situ high throughput synthesis of high-entropy alloys[J]. Scripta Materialia, 2019, 160: 44-47. DOI:10.1016/j.scriptamat.2018.09.040 |
| [78] |
HU Q, GUO S, WANG J M, et al. Parametric study of amorphous high-entropy alloys formation from two new perspectives: atomic radius modification and crystalline structure of alloying elements[J]. Scientific Reports, 2017, 7: 39917. DOI:10.1038/srep39917 |
| [79] |
吴佳琦, 孙奕韬, 汪卫华, 等. 机器学习在非晶材料中的应用[J]. 中国科学:物理学 力学 天文学, 2020, 50(6): 7-20. WU J Q, SUN Y T, WANG W H, et al. Application of machine learning approach in disordered materials[J]. SCIENTIA SINICA Physica, Mechanica & Astronomica, 2020, 50(6): 7-20. |
| [80] |
WEN C, ZHANG Y, WANG C X, et al. Machine learning assisted design of high entropy alloys with desired property[J]. Acta Materialia, 2019, 170: 109-117. DOI:10.1016/j.actamat.2019.03.010 |
| [81] |
DAI D B, XU T, WEI X, et al. Using machine learning and feature engineering to characterize limited material datasets of high-entropy alloys[J]. Computational Materials Science, 2020, 175: 109618. DOI:10.1016/j.commatsci.2020.109618 |
 2021, Vol. 49
2021, Vol. 49